Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (3): 536-541. doi: 10.19723/j.issn.1671-167X.2019.03.024
Previous Articles Next Articles
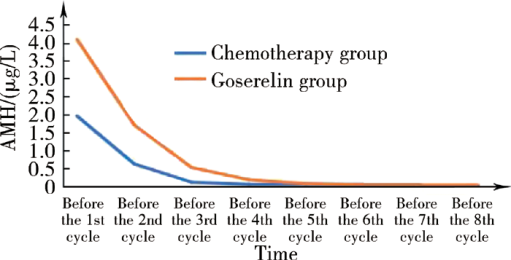
Anti-Müllerian hormone as a new marker of the ovarian reserve function preservation by goserelin during (neo)adjuvant chemotherapy for young breast cancer patients
- Department of Breast Surgery, Peking University People’s Hospital, Beijing 100044, China
CLC Number:
- R737.9
| [1] |
Smigal C, Jemal A, Ward E , et al. Trends in breast cancer by race and ethnicity: update 2006[J]. CA Cancer J Clin, 2006,56(3):168-183.
doi: 10.3322/canjclin.56.3.168 |
| [2] |
Ganz PA, Hahn EE . Implementing a survivorship care plan for patients with breast cancer[J]. J Clin Oncol, 2008,26(5):759-767.
doi: 10.1200/JCO.2007.14.2851 |
| [3] |
Warne GL, Fairley KF, Hobbs JB , et al. Cyclophosphamide-induced ovarian failure[J]. N Engl J Med, 1973,289(22):1159-1162.
doi: 10.1056/NEJM197311292892202 |
| [4] |
Anderson RA, Themmen AP, Al-Qahtani A , et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer[J]. Hum Reprod, 2006,21(10):2583-2592.
doi: 10.1093/humrep/del201 |
| [5] |
Partridge AH, Gelber S, Peppercorn J , et al. Fertility and menopausal outcomes in young breast cancer survivors[J]. Clin Breast Cancer, 2008,8(1):65-69.
doi: 10.3816/CBC.2008.n.004 |
| [6] |
Schover LR . Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility[J]. J Clin Oncol, 2008,26(5):753-758.
doi: 10.1200/JCO.2007.14.1655 |
| [7] |
Del Mastro L, Catzeddu T, Boni L , et al. Prevention of chemotherapy-induced meno-pause by temporary ovarian suppression with goserelin in young, early breast cancer patients[J]. Ann Oncol, 2006,17(1):74-78.
doi: 10.1093/annonc/mdj029 |
| [8] |
Moore HC, Unger JM, Phillips KA , et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy[J]. N Engl J Med, 2015,372(10):923-932.
doi: 10.1056/NEJMoa1413204 |
| [9] | Lambertini M, Ceppi M, Poggio F , et al. Ovarian suppression using luteinizing hormone releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies[J]. Ann Oncol, 2015,26(12):2408-2419. |
| [10] |
Lambertini M, Moore H, Leonard R , et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data[J]. J Clin Oncol, 2018,36(19):1981-1990.
doi: 10.1200/JCO.2018.78.0858 |
| [11] | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, Version 2. 2013 [S/OL]. ( 2013-03-11)[2019-03-01].https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. |
| [12] |
Sun W, Stegmann BJ, Henne M , et al. A new approach to ovarian reserve testing[J]. Fertil Steril, 2008,90(6):2196-2202.
doi: 10.1016/j.fertnstert.2007.10.080 |
| [13] |
La Marca A, Broekmans FJ, Volpe A , et al. Anti-Müllerian hormone (AMH): what do we still need to know[J]. Hum Reprod, 2009,24(9):2264-2275.
doi: 10.1093/humrep/dep210 |
| [14] |
Himabindu Y, Sriharibabu M, Gopinathan K , et al. Anti-Müllerian hormone and antral follicle count as predictors of ovarian response in assisted reproduction[J]. J Hum Reprod Sci, 2013,6(1):27-31.
doi: 10.4103/0974-1208.112377 |
| [15] | Del Mastro L, Boni L, Michelotti A , et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer[J]. JAMA, 2011,306(3):269-276. |
| [16] |
Gerber B, von Minckwitz G, Stehle H , et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study[J]. J Clin Oncol, 2011,29(17):2334-2341.
doi: 10.1200/JCO.2010.32.5704 |
| [17] |
Munster PN, Moore AP, Ismailkhan R , et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo) adjuvant chemotherapy for breast cancer[J]. J Clin Oncol, 2012,30(5):533-538.
doi: 10.1200/JCO.2011.34.6890 |
| [1] | Yun-jing ZHANG,Li-ying QIAO,Meng QI,Ying YAN,Wei-wei KANG,Guo-zhen LIU,Ming-yuan WANG,Yun-feng XI,Sheng-feng WANG. Development and validation of risk prediction model for new-onset cardiovascular diseases among breast cancer patients: Based on regional medical data of Inner Mongolia [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 471-479. |
| [2] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [3] | Yue WANG,Shuang ZHANG,Hong ZHANG,Li LIANG,Ling XU,Yuan-jia CHENG,Xue-ning DUAN,Yin-hua LIU,Ting LI. Clinicopathological features and prognosis of hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 853-862. |
| [4] | Guo-hong SONG,Hui-ping LI,Li-jun DI,Ying YAN,Han-fang JIANG,Ling XU,Dong-gui WAN,Ying LI,Mo-pei WANG,Yu XIAO,Ru-yan ZHANG,Ran RAN,Huan WANG. Efficacy and safety of oral pyrotinib in HER2 positive metastatic breast cancer: real-world practice [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 254-260. |
| [5] | LI Xiu-nan, LIU Ai-hui, TANG Xin, REN Yu. Urothelial carcinoma-associated 1 enhances tamoxifen resistance in breast cancer cells through competitively inhibiting miR-18a [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 295-302. |
| [6] | SHAO Bin, LI Hui-ping, DI Li-jun, SONG Guo-hong, JIANG Han-fang, LIANG Xu, WANG Chao-ying, YAN Ying, LIN Xiao-lin, WANG Li-na, WAN Feng-ling, YUAN Yan-hua, YOU Miao-ning. Predictive and prognostic value of monitoring lymphocyte subsets in peripheral blood before and after chemotherapy in patients with metastatic breast cancer [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 304-309. |
|
||