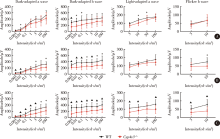
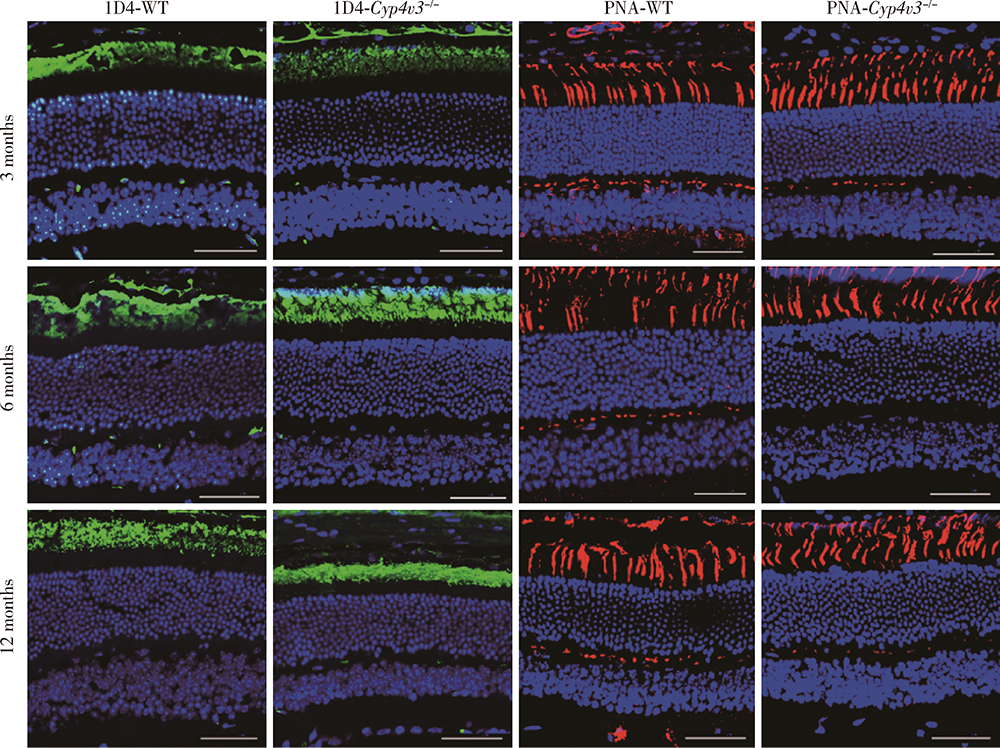
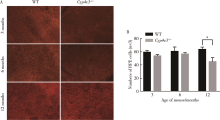
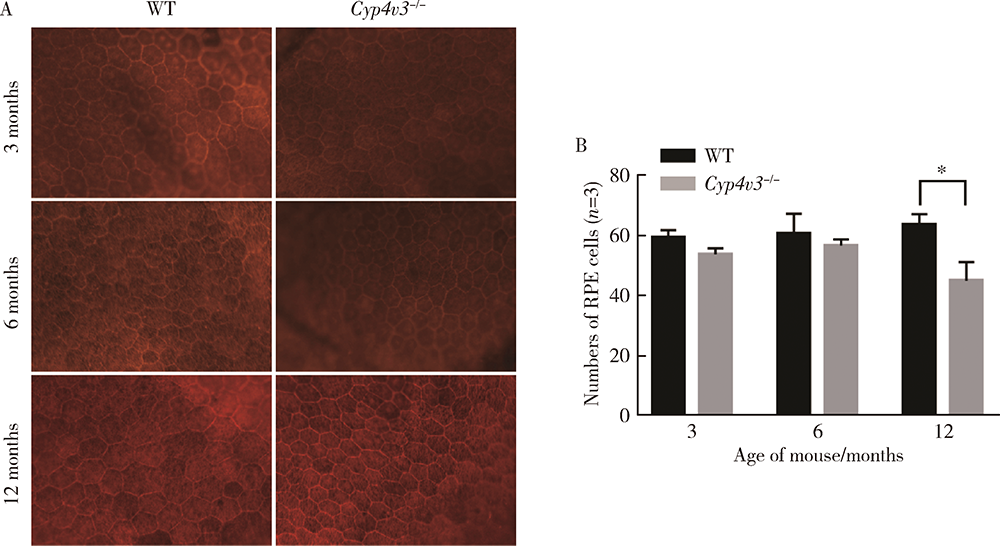
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1099-1106. doi: 10.19723/j.issn.1671-167X.2021.06.016
Previous Articles Next Articles
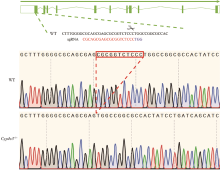
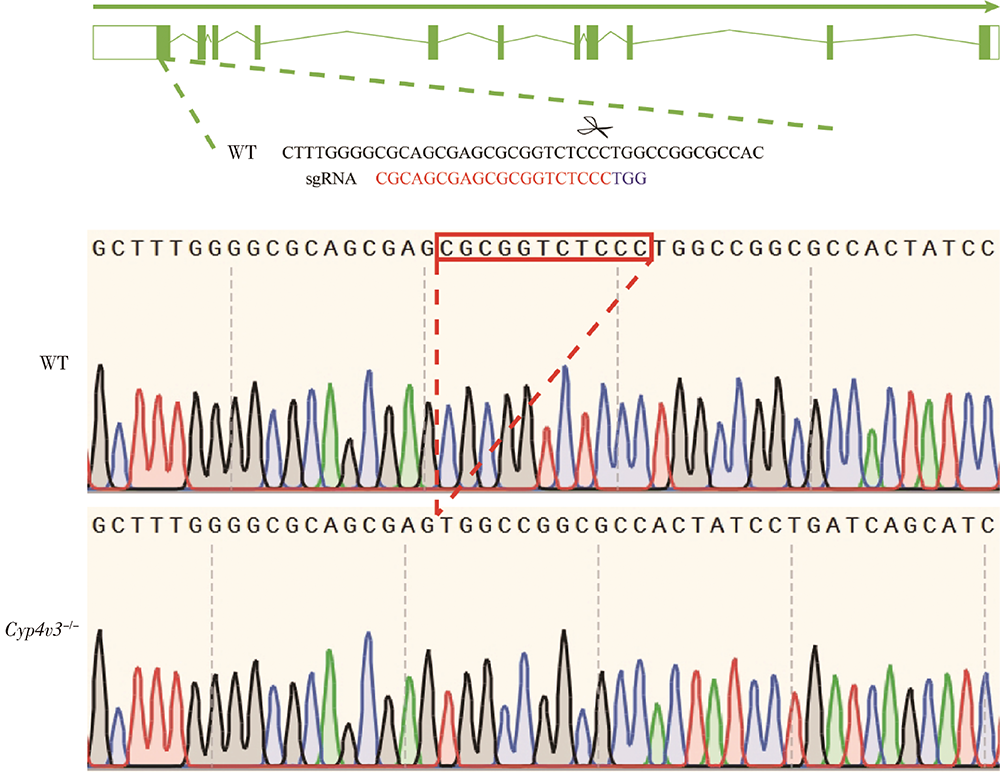
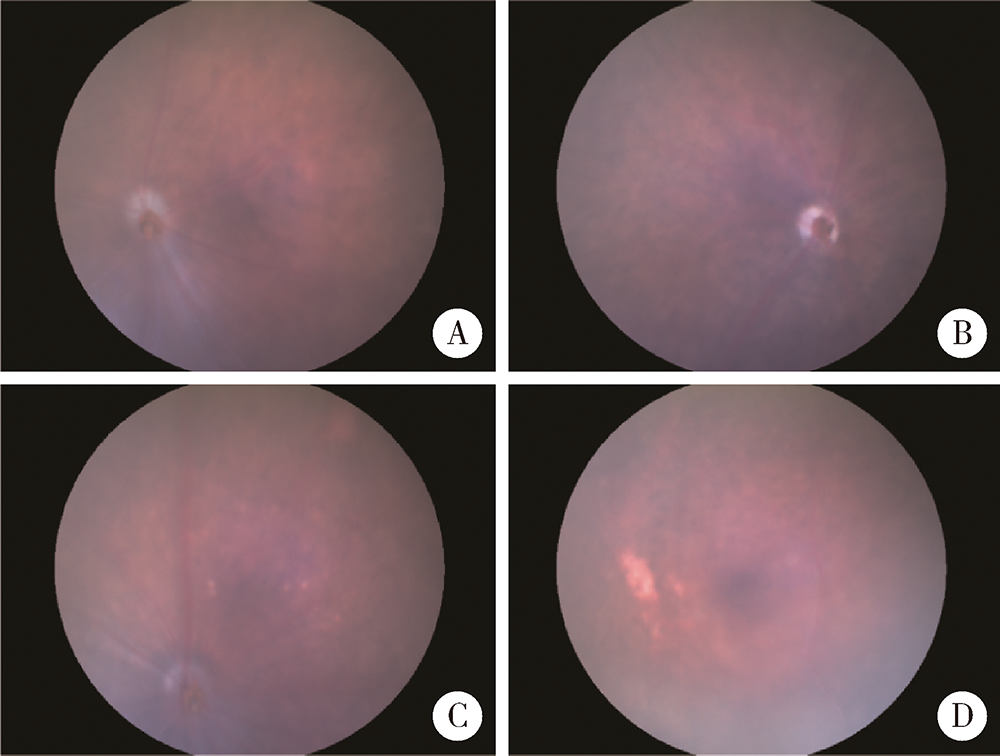
Generation and characterization of Cyp4v3 gene knockout mice
JIA Rui-xuan,JIANG Shang-wei,ZHAO Lin,YANG Li-ping( )
)
- Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Beijing 100191, China
CLC Number:
- R774.1
| [1] | Vargas M, Mitchell A, Yang P, et al. Bietti crystalline dystrophy[M]. Seattle: University of Washington,Seattle, 2012: 12. |
| [2] | Bietti G. Ueber familiaeres Vorkommen von ‘Retinitis punctata albescens’ (verbunden mit ‘Dystrophia marginalis cristallinea corneae’), Glitzern des Glaskoerpers und anderen degenerativen Augenveraenderungen[J]. Klin Monatsbl Augenheilkd, 1937, 99:737-745. |
| [3] |
Xiao X, Mai G, Li S, et al. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy[J]. Biochem Biophys Res Commun, 2011, 409(2):181-186.
doi: 10.1016/j.bbrc.2011.04.112 |
| [4] | Meng XH, Guo H, Xu HW, et al. Identification of novel CYP4V2 gene mutations in 92 Chinese families with Bietti’s crystalline corneoretinal dystrophy[J]. Mol Vis, 2014, 20:1806-1814. |
| [5] |
Fong AMY, Koh A, Lee K, et al. Bietti’s crystalline dystrophy in Asians: Clinical, angiographic and electrophysiological charac-teristics[J]. Int Ophthalmol, 2009, 29(6):459-470.
doi: 10.1007/s10792-008-9266-7 |
| [6] |
Kaiser-Kupfer MI, Chan CC, Markello TC, et al. Clinical biochemical and pathologic correlations in Bietti’s crystalline dystrophy[J]. Am J Ophthalmol, 1994, 118(5):569-582.
pmid: 7977570 |
| [7] | Yuzawa M, Mae Y, Matsui M. Bietti’s crystalline retinopathy[J]. Ophthalmic Genetics, 1986, 7(1):9-20. |
| [8] |
Wilson DJ, Weleber RG, Klein ML, et al. Bietti’s crystalline dystrophy: A clinicopathologic correlative study[J]. Arch Ophthalmol, 1989, 107(2):213-221.
doi: 10.1001/archopht.1989.01070010219026 |
| [9] |
Bernauer W, Daicker B. Bietti’s corneal-retinal dystrophy: A 16-year progression[J]. Retina, 1992, 12(1):18-20.
doi: 10.1097/00006982-199212010-00004 |
| [10] |
Rossi S, Testa F, Li A, et al. Clinical and genetic features in Italian Bietti crystalline dystrophy patients[J]. Br J Ophthalmol, 2013, 97(2):174-179.
doi: 10.1136/bjophthalmol-2012-302469 |
| [11] |
Toto L, Carpineto P, Parodi MB, et al. Spectral domain optical coherence tomography and in vivo confocal microscopy imaging of a case of Bietti’s crystalline dystrophy[J]. Clin Exp Optom, 2013, 96(1):39-45.
doi: 10.1111/j.1444-0938.2012.00784.x |
| [12] | Saatci AO, Doruk HC, Yaman A, et al. Spectral domain optical coherence tomographic findings of Bietti crystalline dystrophy[J]. J Ophthalmol, 2014, 2014:739271. |
| [13] | Özkiriş A, Evereklioğlu C, et al. A comparison of electroretinographic values of patients with Bietti’s crystalline dystrophy with normal individuals[J]. Erciyes Tip Dergisi, 2004, 26(3):113-118. |
| [14] |
Lai TY, Ng TK, Tam PO, et al. Genotype phenotype analysis of Bietti’s crystalline dystrophy in patients with CYP4V2 mutations[J]. Invest Ophthalmol Vis Sci, 2007, 48(11):5212-5220.
doi: 10.1167/iovs.07-0660 |
| [15] |
Mansour AM, Uwaydat SH, Chan CC. Long-term follow-up in Bietti crystalline dystrophy[J]. Eur J Ophthalmol, 2007, 17(4):680-682.
pmid: 17671952 |
| [16] |
Li A, Jiao X, Munier FL, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2[J]. Am J Hum Genet, 2004, 74(5):817-826.
doi: 10.1086/383228 |
| [17] | Shan M, Dong B, Zhao X, et al. Novel mutations in the CYP4V2 gene associated with Bietti crystalline corneoretinal dystrophy[J]. Mol Vis, 2005, 11:738-743. |
| [18] |
Yin H, Jin C, Fang X, et al. Molecular analysis and phenotypic study in 14 Chinese families with Bietti crystalline dystrophy[J]. PLoS One, 2014, 9(4):e94960.
doi: 10.1371/journal.pone.0094960 |
| [19] |
Yin X, Yang L, Chen N, et al. Identification of CYP4V2 mutation in 36 Chinese families with Bietti crystalline corneoretinal dystrophy[J]. Exp Eye Res, 2016, 146:154-162.
doi: 10.1016/j.exer.2016.03.007 |
| [20] |
Darki F, Fekri S, Farhangmehr S, et al. CYP4V2 mutation screening in an Iranian Bietti crystalline dystrophy pedigree and evidence for clustering of CYP4V2 mutations[J]. J Curr Ophthalmol, 2019, 31(2):172-179.
doi: 10.1016/j.joco.2019.01.007 |
| [21] |
Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase[J]. Drug Metab Dispos, 2009, 37(11):2119-2122.
doi: 10.1124/dmd.109.028530 |
| [22] |
Lai TY, Chu KO, Chan KP, et al. Alterations in serum fatty acid concentrations and desaturase activities in Bietti crystalline dystrophy unaffected by CYP4V2 genotypes[J]. Invest Ophthalmol Vis Sci, 2010, 51(2):1092-1097.
doi: 10.1167/iovs.09-3665 |
| [23] |
Kumar S. Comparative modeling and molecular docking of orphan human CYP4V2 protein with fatty acid substrates: Insights into substrate specificity[J]. Bioinformation, 2011, 7(7):360-365.
doi: 10.6026/bioinformation |
| [24] |
Lockhart CM, Smith TB, Yang P, et al. Longitudinal characterisation of function and structure of Bietti crystalline dystrophy: Report on a novel homozygous mutation in CYP4V2[J]. Br J Ophthalmol, 2018, 102(2):187-194.
doi: 10.1136/bjophthalmol-2016-309696 |
| [25] |
Lockhart CM, Nakano M, Rettie AE, et al. Generation and characterization of a murine model of Bietti crystalline dystrophy[J]. Invest Ophthalmol Vis Sci, 2014, 55(9):5572-5581.
doi: 10.1167/iovs.13-13717 |
| [26] |
Hirashima T, Miyata M, Ishihara K, et al. Choroidal vasculature in Bietti crystalline dystrophy with CYP4V2 mutations and in retinitis pigmentosa with EYS mutations[J]. Invest Ophthalmol Vis Sci, 2017, 58(10):3871-3878.
doi: 10.1167/iovs.17-21515 |
| [27] |
Xiong W, Wu DM, Xue Y, et al. AAV cis-regulatory sequences are correlated with ocular toxicity[J]. Proc Natl Acad Sci USA, 2019, 116(12):5785-5794.
doi: 10.1073/pnas.1821000116 |
| [28] |
Strauss O. The retinal pigment epithelium in visual function[J]. Physiol Rev, 2005, 85(3):845-881.
pmid: 15987797 |
| [29] |
Rando RR. The Biochemistry of the visual cycle[J]. Chem Rev, 2001, 101(7):1881-1896.
pmid: 11710234 |
| [30] |
Nakano M, Kelly EJ, Wiek C, et al. CYP4V2 in Bietti’s crystalline dystrophy: Ocular localization, metabolism of omega-3-polyunsaturated fatty acids, and functional deficit of the p.H331P variant[J]. Mol Pharmacol, 2012, 82(4):679-686.
doi: 10.1124/mol.112.080085 |
| [31] |
Hata M, Ikeda HO, Iwai S, et al. Reduction of lipid accumulation rescues Bietti’s crystalline dystrophy phenotypes[J]. Proc Natl Acad Sci USA, 2018, 115(15):3936-3941.
doi: 10.1073/pnas.1717338115 |
| [1] | Jing ZHANG,Jia-gui SONG,Zhen-bin WANG,Yu-qing GONG,Tian-zhuo WANG,Jin-yu ZHOU,Jun ZHAN,Hong-quan ZHANG. Kindlin-2 regulates endometrium development via mTOR and Hippo signaling pathways in mice [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 846-852. |
| [2] | Xiao-wei ZHANG,Hua-qi YIN,Qing LI,Yong-ping ZHAO,BRANDES Kite,Wen-jun BAI,Tao XU. CMTM2 is involved in spermiogenesis in mice [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 228-233. |
|
||