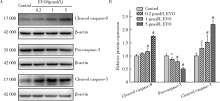
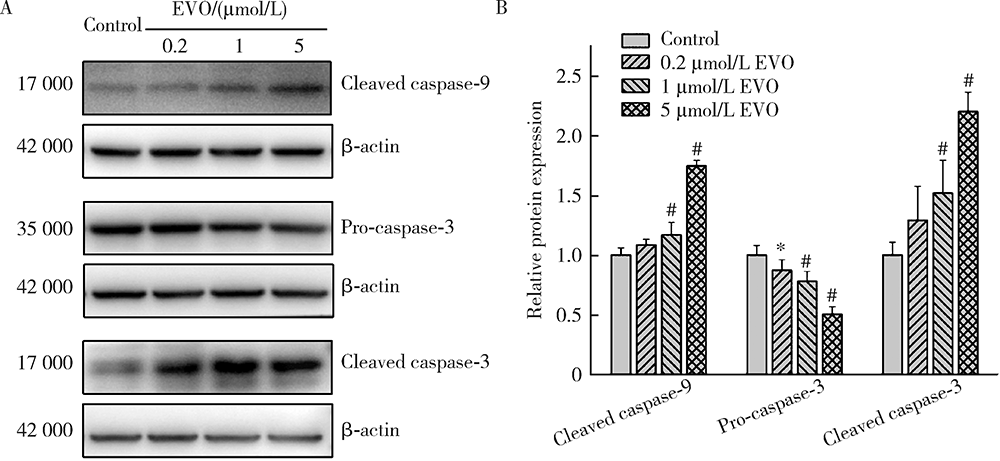
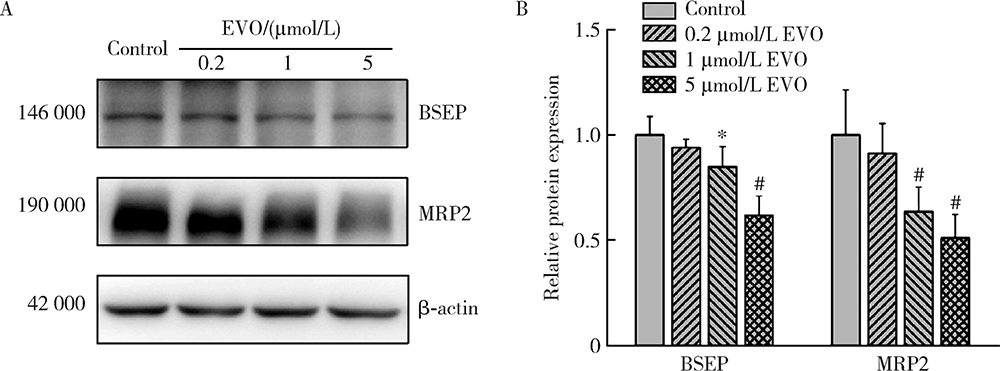
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1107-1114. doi: 10.19723/j.issn.1671-167X.2021.06.017
Previous Articles Next Articles
Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells
GAO Ya-dong1,ZHU An1,LI Lu-di1,ZHANG Tao1,WANG Shuo1,SHAN Dan-ping1,LI Ying-zi1,WANG Qi1,2,3,△( )
)
- 1. Department of Toxicology, Peking University School of Public Health, Beijing 100191, China
2. Key Laboratory of State Administration of Traditional Chinese Medicine for Compatibility Toxicology, Beijing 100191, China
3. Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
CLC Number:
- R114
| [1] | 国家药典委员会. 中华人民共和国药典一部[M]. 11版. 北京: 中国医药科技出版社, 2020: 178. |
| [2] | 刘颖, 杨润芳, 夏祺悦, 等. 吴茱萸醇提物重复给药的靶器官毒性研究[J]. 现代预防医学, 2015, 42(14):2600-2603. |
| [3] | 黄伟, 李晓骄阳, 孙蓉. 吴茱萸水提组分多次给药对小鼠肝毒性的“量-时-毒”关系研究[J]. 中国中药杂志, 2012, 37(15):2223-2227. |
| [4] |
Wang L, Fang K, Cheng J, et al. Scaffold hopping of natural product evodiamine: discovery of a novel antitumor scaffold with excellent potency against colon cancer[J]. J Med Chem, 2020, 63(2):696-713.
doi: 10.1021/acs.jmedchem.9b01626 pmid: 31880942 |
| [5] |
Meng T, Fu S, He D, et al. Evodiamine inhibits lipopolysaccharide (LPS)-induced inflammation in BV-2 cells via regulating AKT/Nrf2-HO-1/NF-κB signaling axis[J]. Cell Mol Neurobiol, 2020, 41(1):115-127.
doi: 10.1007/s10571-020-00839-w |
| [6] |
Wu JY, Chang MC, Chen CS, et al. Topoisomerase Ⅰ inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae[J]. Planta Med, 2013, 79(1):27-29.
doi: 10.1055/s-00000058 |
| [7] |
Lin J, Zhang X, Li C, et al. Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response[J]. J Ethnopharmacol, 2020, 254:112727.
doi: 10.1016/j.jep.2020.112727 |
| [8] |
Zhang Y, Wang J, Wang C, et al. Pharmacological basis for the use of evodiamine in Alzheimer’s disease: antioxidation and antiapoptosis[J]. Int J Mol Sci, 2018, 19(5):1527.
doi: 10.3390/ijms19051527 |
| [9] | Li F, Dong YZ, Zhang D, et al. Molecular mechanisms involved in drug-induced liver injury caused by urate-lowering Chinese herbs: a network pharmacology study and biology experiments[J]. PLoS One, 2019, 14(5):e216948. |
| [10] | 黄伟, 孙蓉. 吴茱萸水提组分多次给药致小鼠肝毒性氧化损伤机制研究[J]. 中药药理与临床, 2012, 28(5):114-116. |
| [11] | 蔡卿嫣. 吴茱萸水提物的大鼠肝毒性及其线粒体损伤机制研究[D]. 广州: 广州中医药大学, 2014. |
| [12] |
Tolosa L, Gómez-Lechón MJ, Pérez-Cataldo G, et al. HepG2 cells simultaneously expressing five P450 enzymes for the screening of hepatotoxicity: identification of bioactivable drugs and the potential mechanism of toxicity involved[J]. Arch Toxicol, 2013, 87(6):1115-1127.
doi: 10.1007/s00204-013-1012-x pmid: 23397584 |
| [13] |
Jain AN. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine[J]. J Med Chem, 2003, 46(4):499-511.
doi: 10.1021/jm020406h |
| [14] | 李文兰, 孙向明, 陈晨, 等. 基于UPLC-Q-TOF MS的吴茱萸致肝毒性部位及入血成分分析[J]. 质谱学报, 2017, 38(3):282-293. |
| [15] | 高绪聪, 柴振海, 张宗鹏. 药物性肝损伤的生物标志物及其评价的研究进展[J]. 中国药理学与毒理学杂志, 2012, 5(26):692-696. |
| [16] |
Huang P, Feng L, Oldham EA, et al. Superoxide dismutase as a target for the selective killing of cancer cells[J]. Nature, 2000, 407(6802):390-395.
doi: 10.1038/35030140 |
| [17] |
Ho E, Karimi Galougahi K, Liu C, et al. Biological markers of oxidative stress: applications to cardiovascular research and practice[J]. Redox Biol, 2013, 1(1):483-491.
doi: 10.1016/j.redox.2013.07.006 |
| [18] | 刘晓婷, 王延让, 张明. 线粒体介导细胞凋亡的研究进展[J]. 环境与健康杂志, 2013, 30(2):182-185. |
| [19] |
Perez MJ, Briz O. Bile-acid-induced cell injury and protection[J]. World J Gastroenterol, 2009, 15(14):1677-1689.
doi: 10.3748/wjg.15.1677 |
| [20] |
Pauli-Magnus C, Stieger B, Meier Y, et al. Enterohepatic transport of bile salts and genetics of cholestasis[J]. J Hepatol, 2005, 43(2):342-357.
pmid: 15975683 |
| [21] |
Yang K, Woodhead JL, Watkins PB, et al. Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated Troglitazone hepatotoxicity[J]. Clin Pharmacol Ther, 2014, 96(5):589-598.
doi: 10.1038/clpt.2014.158 pmid: 25068506 |
| [22] |
Fattinger K. The endothelin antagonist bosentan inhibits the cana-licular bile salt export pump: a potential mechanism for hepatic adverse reactions[J]. Clin Pharmacol Ther, 2001, 69(4):223-231.
pmid: 11309550 |
| [23] | Kenna JG. Current concepts in drug-induced bile salt export pump (BSEP) interference[J]. Curr Protoc Toxicol, 2014, 61(1):1-15. |
| [1] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [2] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [3] | Lei-zhen SU,Jie CHEN,Xian LI,Ping JI. Effects of salinomycin on proliferation and apoptosis of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 902-906. |
| [4] | Liang GENG,Jing LV,Jing FAN. Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 247-253. |
| [5] | LI Man, LI Yuan, SUN Lin, SONG Jun-lai, LV Cong. High mobility group box 1 promotes apoptosis of astrocytes after oxygen glucose deprivation/reoxygenation by regulating the expression of Bcl-2 and Bax [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 785-791. |
| [6] | SUN Jing, SONG Wei-dong, YAN Si-yuan, XI Zhi-jun. Chloroquine inhibits viability of renal carcinoma cells and enhances sunitinib-induced caspase-dependent apoptosis [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 778-784. |
| [7] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [8] | WANG Yu-jie, GUO Xiang-yang, WANG Jun. Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 310-314. |
| [9] | YANG Guang, CHENG Qing-li, LI Chun-lin, JIA Ya-li, YUE Wen, PEI Xue-tao, LIU Yang, ZHAO Jia-hui, DU Jing, AO Qiang-guo. High glucose reduced the repair function of kidney stem cells conditional medium to the hypoxia-injured renal tubular epithelium cells [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 125-130. |
| [10] | CAO Pei, JIANG Xue-jun, XI Zhi-jun. Sunitinib induces autophagy via suppressing Akt/mTOR pathway in renal cell carcinoma [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 584-589. |
| [11] | LI Gang, ZHANG Hong-xian, WANG Yun-peng, ZHANG Jing,HONG Kai, TIAN Xiao-jun, MA Lu-lin. Protective effect of phloroglucinol on renal ischemia and reperfusion injury [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 743-748. |
| [12] | ZHENG Shao-Qiang, CHEN Xue, WANG Ya-Jie, AN Li-Xin. Effects of sevoflurane on brain neuroapoptosis and ability of long-term learning and memory in newborn rats [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 674-678. |
|
||