Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (5): 971-980. doi: 10.19723/j.issn.1671-167X.2022.05.026
Previous Articles Next Articles
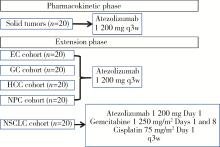
Atezolizumab therapy in Chinese patients with locally advanced or metastatic solid tumors: An open-label, phase Ⅰ study
Li ZHANG1,Ji-fang GONG2,Hong-ming PAN3,Yu-xian BAI4,Tian-shu LIU5,Ying CHENG6,Ya-chi CHEN7,Jia-ying HUANG8,Ting-ting XU8,Fei-jiao GE8,Wan-ling HSU9,Jane SHI10,Xi-chun HU11,*( ),Lin SHEN2,*(
),Lin SHEN2,*( )
)
- 1. Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou 510060, China
2. Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
3. Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310020, China
4. Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin 150081, China
5. Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai 200032, China
6. Department of Medical Oncology, Jilin Cancer Hospital, Changchun 130012, China
7. Clinical Pharmacology, Genentech, Inc., South San Francisco, CA 94080, USA
8. Oncology, Roche Product Development Shanghai, Shanghai, 201203, China
9. Department of Statistics, Roche Product Development Shanghai, Shanghai 201203, China
10. Portfolio Clinical Safety, Roche Product Development Shanghai, Shanghai 201203, China
11. Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China
CLC Number:
- R730.51
| 1 | International Agency for Research on Cancer. China (Globocan 2020)[EB/OL]. (2021-03)[2021-05-15] https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. |
| 2 |
Arnold M , Soerjomataram I , Ferlay J , et al. Global incidence of oesophageal cancer by histological subtype in 2012[J]. Gut, 2015, 64 (3): 381- 387.
doi: 10.1136/gutjnl-2014-308124 |
| 3 | Salehiniya H , Mohammadian M , Mohammadian-Hafshejani A , et al. Nasopharyngeal cancer in the world: Epidemiology, incidence, mortality and risk factors[J]. World Cancer Res J, 2018, 5 (1): e1046. |
| 4 | Merck. Highlights of prescribing information. Keytruda® (Pembrolizumab)[EB/OL]. (2014)[2022-05-12]. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. |
| 5 |
Fuchs CS , Doi T , Jang RW , et al. Safety and efficacy of pembro-lizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial[J]. JAMA Oncol, 2018, 4 (5): e180013.
doi: 10.1001/jamaoncol.2018.0013 |
| 6 | Bristol Myers Squibb Company. Highlights of prescribing information. Opdivo (Nivolumab)[EB/OL]. (2014)[2022-05-03]. https://packageinserts.bms.com/pi/pi_opdivo.pdf. |
| 7 |
Ma BBY , Lim WT , Goh BC , et al. Antitumor activity of ni-volumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo clinic phase 2 consortium (NCI-9742)[J]. J Clin Oncol, 2018, 36 (14): 1412- 1418.
doi: 10.1200/JCO.2017.77.0388 |
| 8 | NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 3.2022[EB/OL]. (2022-03-16)[2022-05]. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. |
| 9 |
Herbst RS , Soria JC , Kowanetz M , et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J]. Nature, 2014, 515 (7528): 563- 567.
doi: 10.1038/nature14011 |
| 10 |
Weinstock C , Khozin S , Suzman D , et al. U.S. Food and Drug Administration approval summary: Atezolizumab for metastatic non-small cell lung cance[J]. Clin Cancer Res, 2017, 23 (16): 4534- 4539.
doi: 10.1158/1078-0432.CCR-17-0540 |
| 11 | Genentech. FDA approves Genentech's Tecentriq plus chemothe-rapy (abraxane and carboplatin) for the initial treatment of metastatic non-squamous non-small cell lung cancer[N/OL]. (2019-12-03)[2021-05-03] https://www.gene.com/media/press-releases/14827/2019-12-03/fda-approves-genentechs-tecentriq-plus-c. |
| 12 | F. Hoffmann-La Roche Ltd. European Commission approves Roche's new Tecentriq-based combination therapy as an initial treatment for most common form of advanced lung cancer[N/OL]. (2019-09-06)[2021-05-11] https://www.roche.com/media/releases/med-cor-2019-09-06.htm. |
| 13 | F. Hoffmann-La Roche Ltd. FDA approves Roche's Tecentriq in combination with Avastin for people with the most common form of liver cancer[N/OL]. (2020-06-02)[2021-05-15] https://www.roche.com/media/releases/med-cor-2020-06-02.htm. |
| 14 |
Finn RS , Qin S , Ikeda M , et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382 (20): 1894- 1905.
doi: 10.1056/NEJMoa1915745 |
| 15 | Xu ZY , Brown L , Pan GW , et al. Lifestyle, environmental pollution and lung cancer in cities of Liaoning in northeastern China[J]. Lung Cancer, 1996, 14 (Suppl 1): S149- S160. |
| 16 |
Grenade C , Phelps MA , Villalona-Calero MA . Race and ethnicity in cancer therapy: What have we learned?[J]. Clin Pharmacol Ther, 2014, 95 (4): 403- 412.
doi: 10.1038/clpt.2014.5 |
| 17 | Genentech. Highlights of prescribing information. Tecentriq®(atezolizumab)[EB/OL]. (2016)[2022-05-15]. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf. |
| 18 |
West H , McCleod M , Hussein M , et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2019, 20 (7): 924- 937.
doi: 10.1016/S1470-2045(19)30167-6 |
| 19 |
Lee MS , Ryoo BY , Hsu CH , et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study[J]. Lancet Oncol, 2020, 21 (6): 808- 820.
doi: 10.1016/S1470-2045(20)30156-X |
| 20 |
Zhang T , Xie J , Arai S , et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory can-cers: A meta-analysis[J]. Oncotarget, 2016, 7 (45): 73068- 73079.
doi: 10.18632/oncotarget.12230 |
| 21 |
Le DT , Kavan P , Kim TW , et al. KEYNOTE-164: Pembrolizu-mab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer[J]. J Clin Oncol, 2018, 36 (Suppl 15): 3514.
doi: 10.1200/jco.2018.36.15_suppl.3514 |
| 22 |
Colevas AD , Bahleda R , Braiteh F , et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase Ⅰ trial[J]. Ann Oncol, 2018, 29 (11): 2247- 2253.
doi: 10.1093/annonc/mdy411 |
| 23 |
Schiller JH , Harrington D , Belani CP , et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer[J]. N Engl J Med, 2002, 346 (2): 92- 98.
doi: 10.1056/NEJMoa011954 |
| 24 | International Agency for Research on Cancer. All cancers (Globocan 2020)[EB/OL]. (2020-12)[2022-05-12] https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Yuxuan TIAN,Mingjian RUAN,Yi LIU,Derun LI,Jingyun WU,Qi SHEN,Yu FAN,Jie JIN. Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 567-574. |
| [3] | Kaifeng YAO,Mingjian RUAN,Derun LI,Yuxuan TIAN,Yuke CHEN,Yu FAN,Yi LIU. Diagnostic efficacy of targeted biopsy combined with regional systematic biopsy in prostate cancer in patients with PI-RADS 4-5 [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 575-581. |
| [4] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [5] | Ting JING,Hua JIANG,Ting LI,Qianqian SHEN,Lan YE,Yindan ZENG,Wenxin LIANG,Gang FENG,Man-Yau Szeto Ignatius,Yumei ZHANG. Relationship between serum 25-hydroxyvitamin D and handgrip strength in middle-aged and elderly people in five cities of Western China [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 448-455. |
| [6] | Qingbo WANG,Hongqiao FU. Main characteristics and historical evolution of China' s health financing transition [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 462-470. |
| [7] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [8] | Yi LIU,Chang-wei YUAN,Jing-yun WU,Qi SHEN,Jiang-xi XIAO,Zheng ZHAO,Xiao-ying WANG,Xue-song LI,Zhi-song HE,Li-qun ZHOU. Diagnostic efficacy of prostate cancer using targeted biopsy with 6-core systematic biopsy for patients with PI-RADS 5 [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 812-817. |
| [9] | Min QIU,You-long ZONG,Bin-shuai WANG,Bin YANG,Chu-xiao XU,Zheng-hui SUN,Min LU,Lei ZHAO,Jian LU,Cheng LIU,Xiao-jun TIAN,Lu-lin MA. Treatment outcome of laparoscopic partial nephrectomy in patients with renal tumors of moderate to high complexity [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 833-837. |
| [10] | Chang-wei YUAN,De-run LI,Zhi-hua LI,Yi LIU,Gang-zhi SHAN,Xue-song LI,Li-qun ZHOU. Application of dynamic contrast enhanced status in multiparametric magnetic resonance imaging for prostatic cancer with PI-RADS 4 lesion [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 838-842. |
| [11] | Hui-li LIU,Yan-han LV,Xiao-xiao WANG,Min LI. Factors influencing the chronic post-surgical pain after laparoscopic surgery for elderly patients with urinary tract tumors [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 851-856. |
| [12] | Zhong CAO,Hong-bing CEN,Jian-hong ZHAO,Jun MEI,Ling-zhi QIN,Wei LIAO,Qi-lin AO. Expression and significance of INSM1 and SOX11 in pancreatic neuroendocrine tumor and solid pseudopapillary neoplasm [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 575-581. |
| [13] | Jian-xun MA,You-chen XIA,Bi LI,Hong-mei ZHAO,Yu-tao LEI,Xi BU. Choice of immediate breast reconstructive methods after modified radical mastectomy [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 612-618. |
| [14] | Yu-xiao WU,Yi-fan KANG,Qian-ying MAO,Zi-meng LI,Xiao-feng SHAN,Zhi-gang CAI. Application of methylene blue near-infrared fluorescence imaging for oral sentinel lymph node mapping in rats [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 684-688. |
| [15] | Shang XIE,Zhi-gang CAI,Xiao-feng SHAN. Application value of whole exon sequencing and immune related indicators in the precision treatment of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 697-701. |
|
||