Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (1): 150-156. doi: 10.19723/j.issn.1671-167X.2024.01.023
Previous Articles Next Articles
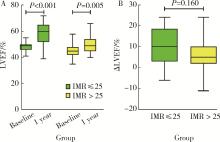
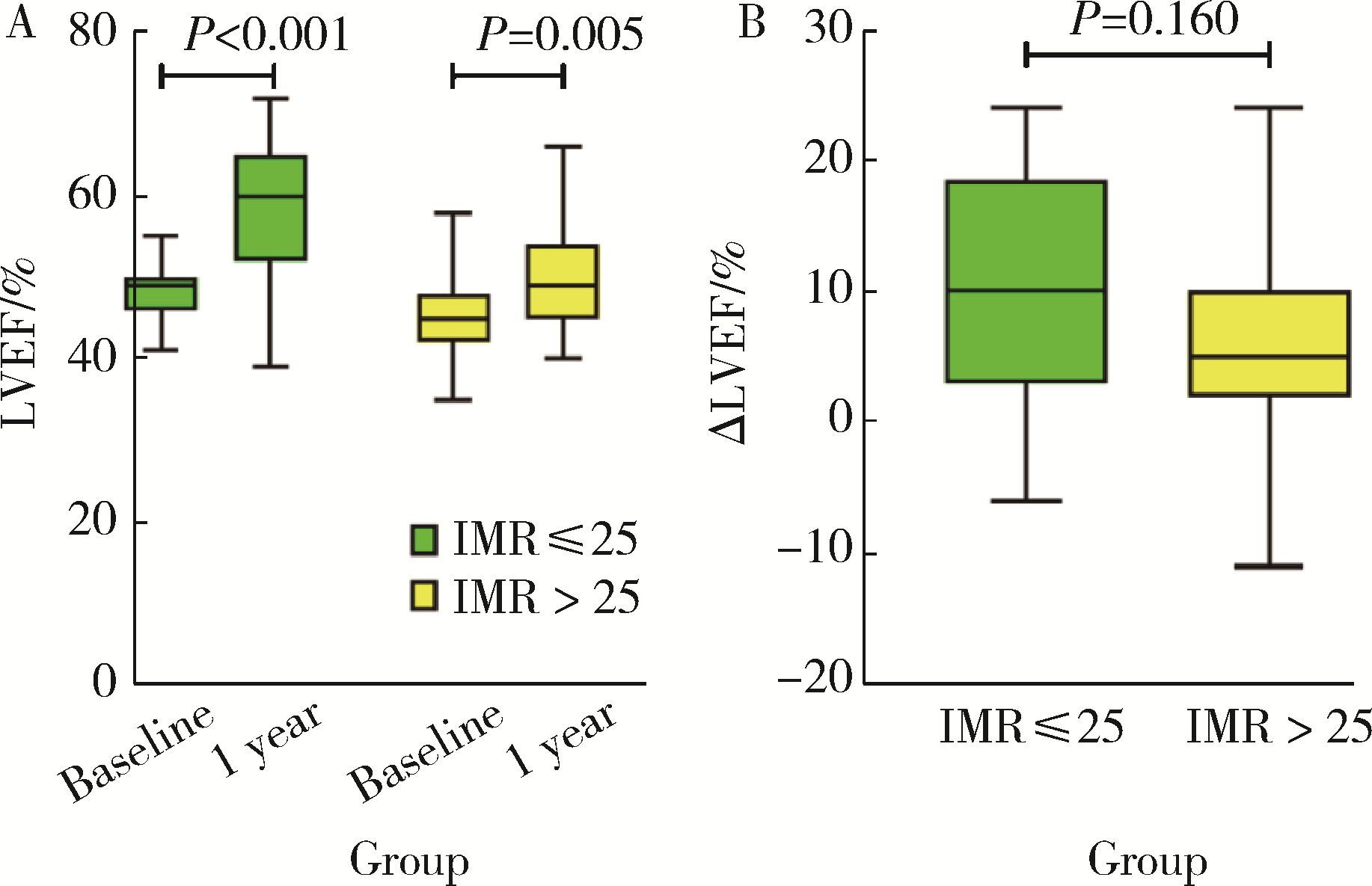
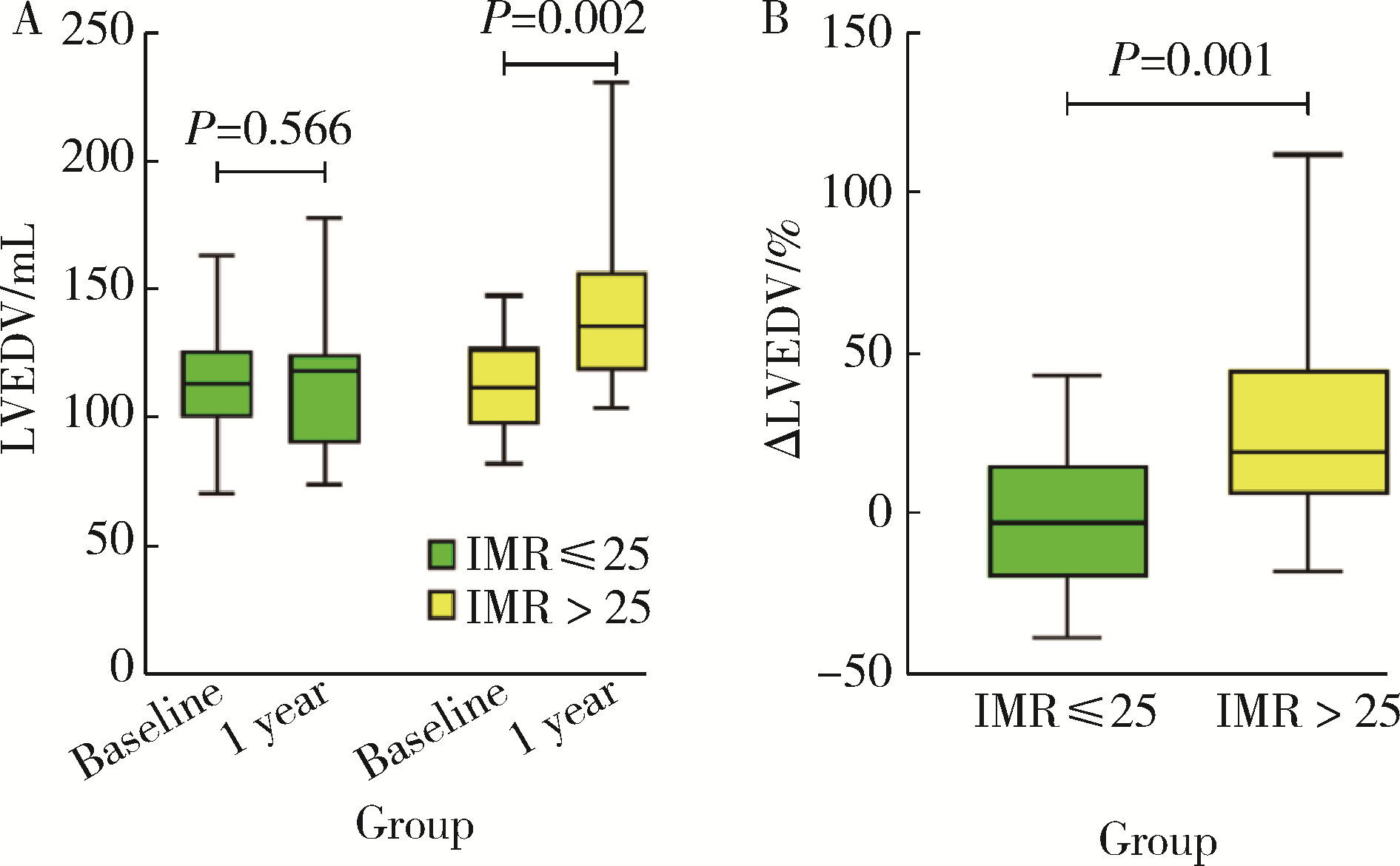
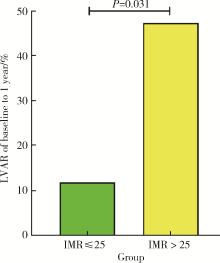
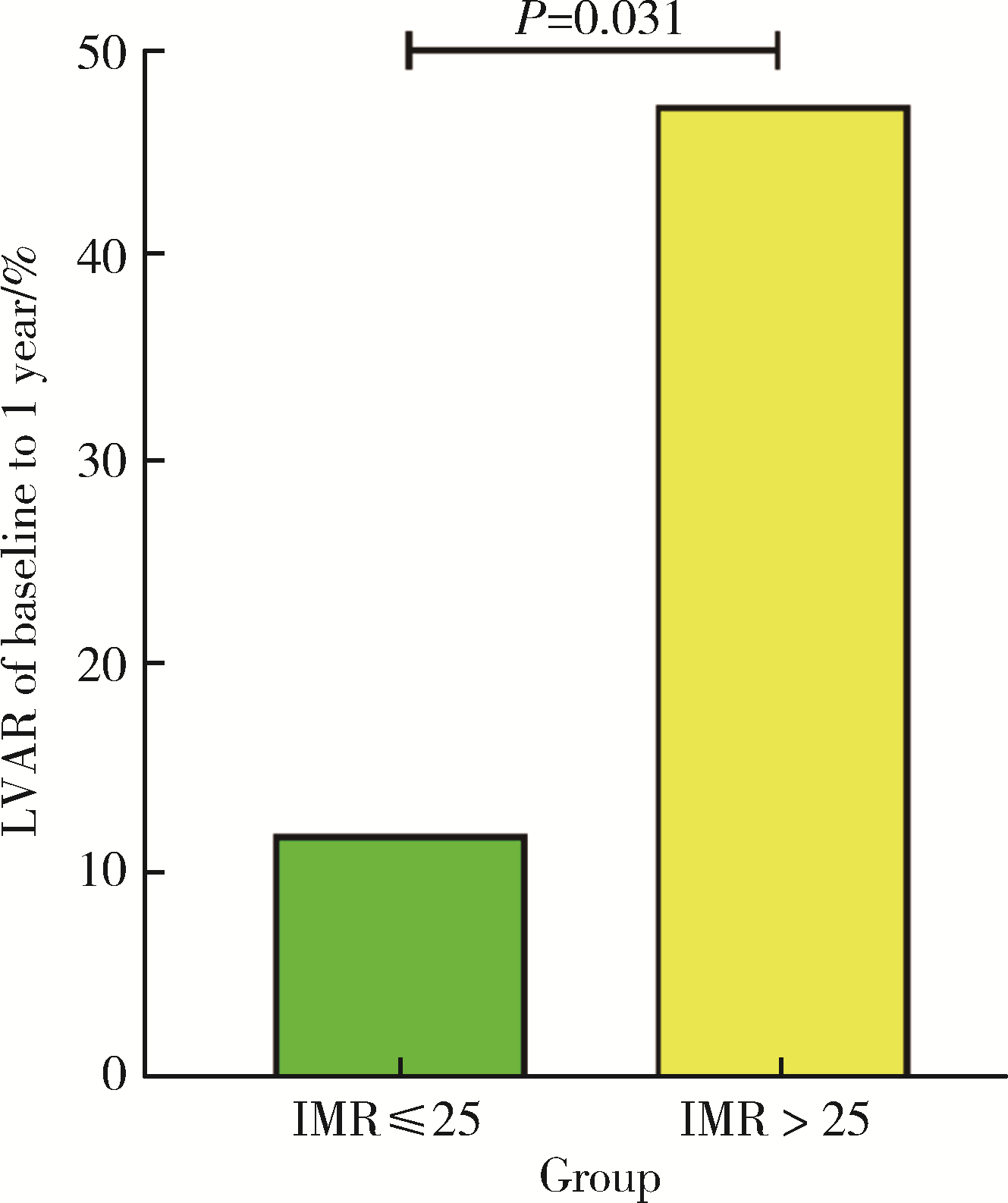
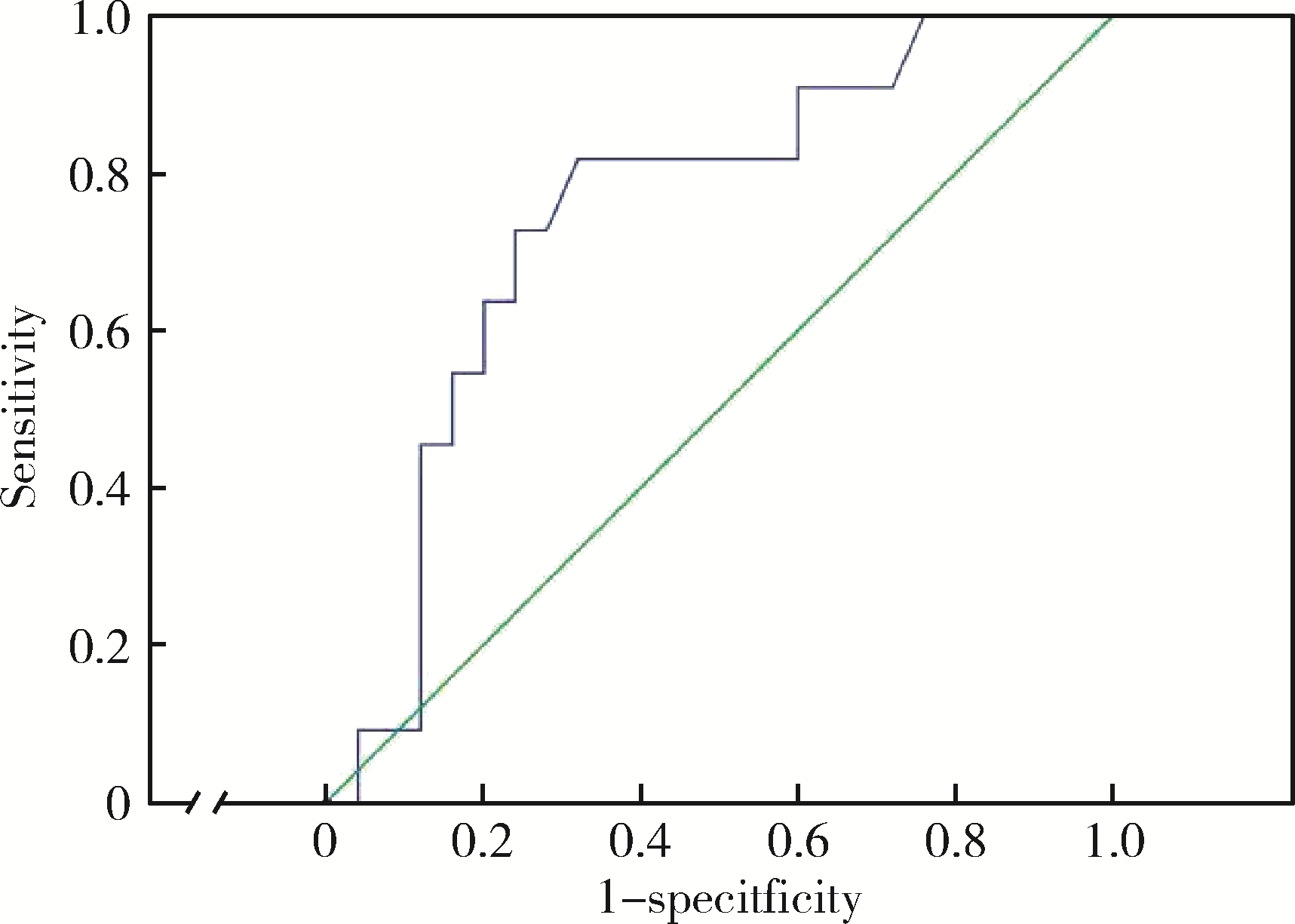
Index of microcirculatory resistance is associated with left ventricular remodeling in patients with acute anterior ST-segment elevation myocardial infarction undergoing emergency primary percutaneous coronary intervention
Fangfang WANG1,Fumeng LIANG2,Nan LI3,Xiaoxiao WANG3,Jiangli HAN1,*( ),Lijun GUO1,*(
),Lijun GUO1,*( )
)
- 1. Department of Cardiology and Institute of Vascular Medicine, Peking University Third Hospital; State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University; NHC Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides, Peking University; Beijing Key Laboratory of Cardiovascular Receptors Research, Beijing 100191, China
2. Department of General Medicine, Peking University Third Hospital, Beifang Branch, Beijing 100089, China
3. Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R542.22
| 1 | Klancik V , Pesl L , Neuberg M , et al. Long-term follow-up in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention[J]. Eur Heart J Suppl, 2022, 24 (Suppl B): B16- B22. |
| 2 |
Araszkiewicz A , Grajek S , Lesiak M , et al. Effect of impaired myocardial reperfusion on left ventricular remodeling in patients with anterior wall acute myocardial infarction treated with primary coronary intervention[J]. Am J Cardiol, 2006, 98 (6): 725- 728.
doi: 10.1016/j.amjcard.2006.04.009 |
| 3 |
杨洋, 李楠, 赖红梅, 等. 急性前壁ST段抬高型心肌梗死患者介入治疗前血清血管内皮生长因子与左心室重构的关联性研究[J]. 中国心血管病研究, 2021, 19 (9): 818- 823.
doi: 10.3969/j.issn.1672-5301.2021.09.012 |
| 4 |
van der Bijl P , Abou R , Goedemans L , et al. Left ventricular post-infarct remodeling: Implications for systolic function improvement and outcomes in the modern era[J]. JACC Heart Fail, 2020, 8 (2): 131- 140.
doi: 10.1016/j.jchf.2019.08.014 |
| 5 |
Ng MK , Yong AS , Ho M , et al. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention[J]. Circ Cardiovasc Interv, 2012, 5 (4): 515- 522.
doi: 10.1161/CIRCINTERVENTIONS.112.969048 |
| 6 |
Yoon GS , Ahn SG , Woo SI , et al. The Index of Microcirculatory resistance after primary percutaneous coronary intervention predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction[J]. J Clin Med, 2021, 10 (20): 4752.
doi: 10.3390/jcm10204752 |
| 7 |
Qi Y , Gu R , Xu J , et al. Index of microcirculatory resistance predicts long term cardiac systolic function in patients with STEMI undergoing primary PCI[J]. BMC Cardiovasc Disord, 2021, 21 (1): 66.
doi: 10.1186/s12872-021-01887-w |
| 8 |
Fearon WF , Low AF , Yong AS , et al. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention[J]. Circulation, 2013, 127 (24): 2436- 2441.
doi: 10.1161/CIRCULATIONAHA.112.000298 |
| 9 |
韩江莉, 何立芸, 崔鸣, 等. 急性心肌梗死直接冠状动脉介入治疗患者微循环阻力指数检测的可行性及临床价值探讨[J]. 中华医学杂志, 2017, 97 (29): 2261- 2265.
doi: 10.3760/cma.j.issn.0376-2491.2017.29.006 |
| 10 |
Akasaka T , Yoshida K , Kawamoto T , et al. Relation of phasic coronary flow velocity characteristics with TIMI perfusion grade and myocardial recovery after primary percutaneous transluminal coronary angioplasty and rescue stenting[J]. Circulation, 2000, 101 (20): 2361- 2367.
doi: 10.1161/01.CIR.101.20.2361 |
| 11 |
Ng MK , Yeung AC , Fearon WF . Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve[J]. Circulation, 2006, 113 (17): 2054- 2061.
doi: 10.1161/CIRCULATIONAHA.105.603522 |
| 12 |
Fearon WF , Balsam LB , Farouque HM , et al. Novel index for invasively assessing the coronary microcirculation[J]. Circulation, 2003, 107 (25): 3129- 3132.
doi: 10.1161/01.CIR.0000080700.98607.D1 |
| 13 |
Aarnoudse W , Fearon WF , Manoharan G , et al. Epicardial stenosis severity does not affect minimal microcirculatory resistance[J]. Circulation, 2004, 110 (15): 2137- 2142.
doi: 10.1161/01.CIR.0000143893.18451.0E |
| 14 |
Berry C , Corcoran D , Hennigan B , et al. Fractional flow reserve-guided management in stable coronary disease and acute myocardial infarction: Recent developments[J]. Eur Heart J, 2015, 36 (45): 3155- 3164.
doi: 10.1093/eurheartj/ehv206 |
| 15 |
van Kranenburg M , Magro M , Thiele H , et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients[J]. JACC Cardiovasc Imaging, 2014, 7 (9): 930- 939.
doi: 10.1016/j.jcmg.2014.05.010 |
| 16 |
Maznyczka AM , McCartney PJ , Oldroyd KG , et al. Risk stratification guided by the index of microcirculatory resistance and left ventricular end-diastolic pressure in acute myocardial infarction[J]. Circ Cardiovasc Interv, 2021, 14 (2): e009529.
doi: 10.1161/CIRCINTERVENTIONS.120.009529 |
| 17 |
Lim HS , Yoon MH , Tahk SJ , et al. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction[J]. Eur Heart J, 2009, 30 (23): 2854- 2860.
doi: 10.1093/eurheartj/ehp313 |
| 18 |
Bolognese L , Neskovic AN , Parodi G , et al. Left ventricular remodeling after primary coronary angioplasty: Patterns of left ventricular dilation and long-term prognostic implications[J]. Circulation, 2002, 106 (18): 2351- 2357.
doi: 10.1161/01.CIR.0000036014.90197.FA |
| 19 |
Shi K , Ma M , Yang MX , et al. Increased oxygenation is associated with myocardial inflammation and adverse regional remodeling after acute ST-segment elevation myocardial Infarction[J]. Eur Radiol, 2021, 31 (12): 8956- 8966.
doi: 10.1007/s00330-021-08032-3 |
| 20 |
Luo E , Wang D , Yan G , et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention[J]. Cardiovasc Diabetol, 2019, 18 (1): 150.
doi: 10.1186/s12933-019-0957-3 |
| 21 |
Garg M , Khanna D , Kalra S , et al. Chronic oral administration of low-dose combination of fenofibrate and rosuvastatin protects the rat heart against experimentally induced acute myocardial infarction[J]. Fundam Clin Pharmacol, 2016, 30 (5): 394- 405.
doi: 10.1111/fcp.12204 |
| 22 | Oidor-Chan VH , Hong E , Perez-Severiano F , et al. Fenofibrate plus metformin produces cardioprotection in a type 2 diabetes and acute myocardial infarction model[J]. PPAR Res, 2016, 2016, 8237264. |
| 23 |
Niccoli G , Scalone G , Lerman A , et al. Coronary microvascular obstruction in acute myocardial infarction[J]. Eur Heart J, 2016, 37 (13): 1024- 1033.
doi: 10.1093/eurheartj/ehv484 |
| 24 |
Ishida K , Geshi T , Nakano A , et al. Beneficial effects of statin treatment on coronary microvascular dysfunction and left ventricular remodeling in patients with acute myocardial infarction[J]. Int J Cardiol, 2012, 155 (3): 442- 447.
doi: 10.1016/j.ijcard.2011.11.015 |
| 25 | Węgiel M , Rakowski T . Circulating biomarkers as predictors of left ventricular remodeling after myocardial infarction[J]. Adv interv cardiol, 2021, 17 (1): 21- 32. |
| [1] | Shengqi ZHENG,Tianchi HUA,Guicao YIN,Wei ZHANG,Ye YAO,Yifan LI. Association between the triglyceride-glucose index and the incidence of nephrolithiasis in male individuals [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 610-616. |
| [2] | Huangda GUO,Hexiang PENG,Siyue WANG,Tianjiao HOU,Yixin LI,Hanyu ZHANG,Mengying WANG,Yiqun WU,Xueying QIN,Xun TANG,Jing LI,Dafang CHEN,Yonghua HU,Tao WU. A ssociations of short-term ambient particulate matter exposure and MTNR1B gene with triglyceride-glucose index: A family-based study [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 375-383. |
| [3] | Qing GAO,Yu CHEN,Gang LIU,Sheng-long CHEN,Sui-xin DONG. Clinical results after surgical treatment for non-selective case with postinfarction ventricular septal rupture [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1103-1107. |
|
||