Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (2): 253-259. doi: 10.19723/j.issn.1671-167X.2024.02.008
Previous Articles Next Articles
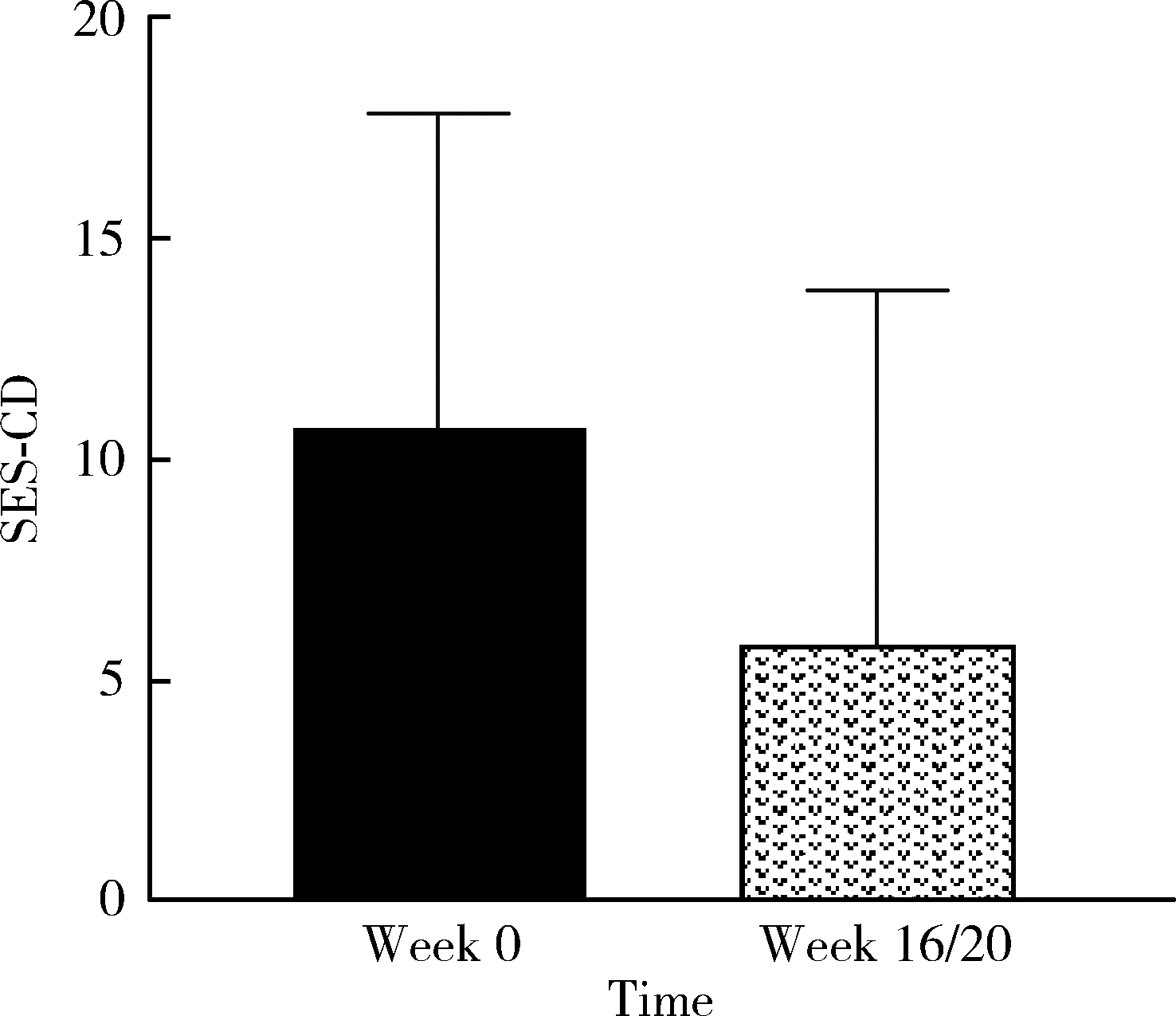
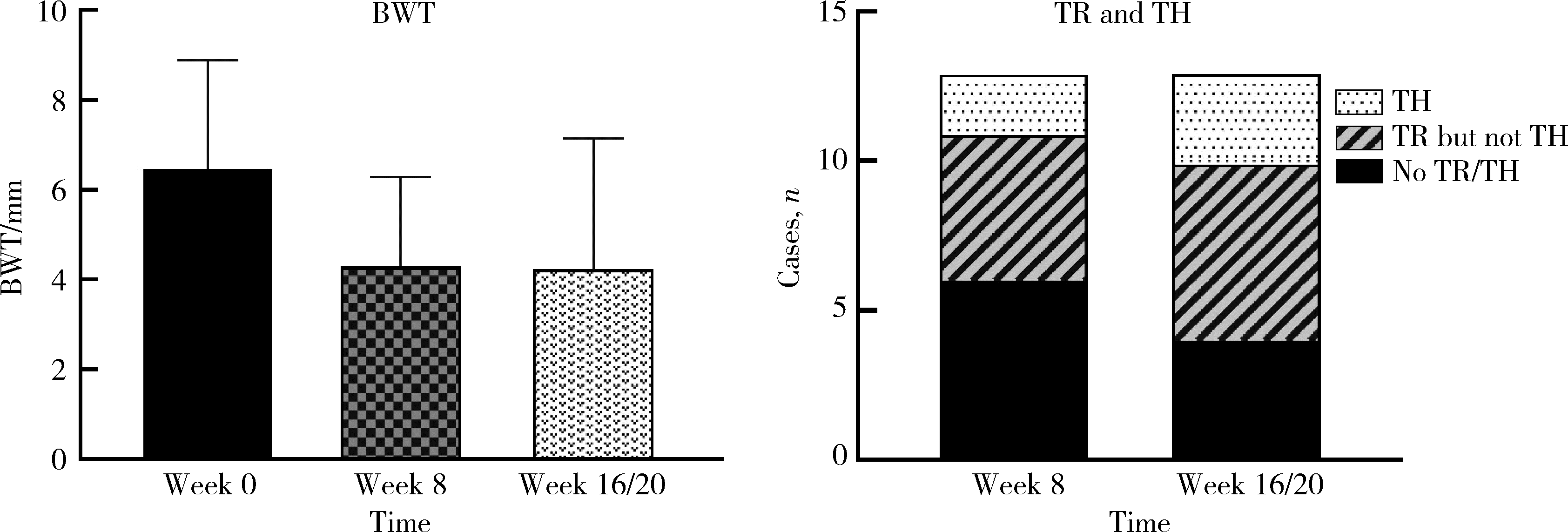
Clinical remission and transmural healing of ustekinumab in patients with Crohn's disease
Yun WU,Yalan XU,Guoyan ZHANG,Yuanyuan ZHANG,Junyao WANG,Peng YOU,Tao PENG,Yulan LIU,Ning CHEN*( )
)
- Department of Gastroenterology, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R574.1
| 1 |
Torres J , Mehandru S , Colombel JF , et al. Crohn's disease[J]. Lancet, 2017, 389 (10080): 1741- 1755.
doi: 10.1016/S0140-6736(16)31711-1 |
| 2 |
Geyl S , Guillo L , Laurent V , et al. Transmural healing as a therapeutic goal in Crohn's disease: A systematic review[J]. Lancet Gastroenterol Hepatol, 2021, 6 (8): 659- 667.
doi: 10.1016/S2468-1253(21)00096-0 |
| 3 |
Helwig U , Fischer I , Hammer L , et al. Transmural response and transmural healing defined by intestinal ultrasound: New potential therapeutic targets?[J]. J Crohns Colitis, 2022, 16 (1): 57- 67.
doi: 10.1093/ecco-jcc/jjab106 |
| 4 |
Best WR , Becktel JM , Singleton JW , et al. Development of a Crohn's disease activity index. National cooperative Crohn's disease study[J]. Gastroenterology, 1976, 70 (3): 439- 444.
doi: 10.1016/S0016-5085(76)80163-1 |
| 5 |
Lu C , Merrill C , Medellin A , et al. Bowel ultrasound state of the art: Grayscale and doppler ultrasound, contrast enhancement, and elastography in Crohn disease[J]. J Ultrasound Med, 2019, 38 (2): 271- 288.
doi: 10.1002/jum.14920 |
| 6 |
Laserna-Mendieta EJ , Lucendo J . Faecal calprotectin in inflammatory bowel diseases: A review focused on meta-analyses and routine usage limitations[J]. Clin Chem Lab Med, 2019, 57 (9): 1295- 1307.
doi: 10.1515/cclm-2018-1063 |
| 7 |
Solem CA , Loftus EJ , Tremaine WJ , et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease[J]. Inflamm Bowel Dis, 2005, 11 (8): 707- 712.
doi: 10.1097/01.MIB.0000173271.18319.53 |
| 8 |
Schnitzler F , Fidder H , Ferrante M , et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease[J]. Inflamm Bowel Dis, 2009, 15 (9): 1295- 1301.
doi: 10.1002/ibd.20927 |
| 9 |
Turner D , Ricciuto A , Lewis A , et al. STRIDE-Ⅱ: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD[J]. Gastroenterology, 2021, 160 (5): 1570- 1583.
doi: 10.1053/j.gastro.2020.12.031 |
| 10 |
Mazzuoli S , Guglielmi FW , Antonelli E , et al. Definition and evaluation of mucosal healing in clinical practice[J]. Dig Liver Dis, 2013, 45 (12): 969- 977.
doi: 10.1016/j.dld.2013.06.010 |
| 11 |
Civitelli F , Nuti F , Oliva S , et al. Looking beyond mucosal hea-ling: Effect of biologic therapy on transmural healing in pediatric Crohn's disease[J]. Inflamm Bowel Dis, 2016, 22 (10): 2418- 2424.
doi: 10.1097/MIB.0000000000000897 |
| 12 |
Zacharopoulou E , Craviotto V , Fiorino G , et al. Targeting the gut layers in Crohn's disease: Mucosal or transmural healing?[J]. Expert Rev Gastroenterol Hepatol, 2020, 14 (9): 775- 787.
doi: 10.1080/17474124.2020.1780914 |
| 13 |
Lafeuille P , Hordonneau C , Vignette J , et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn's disease[J]. Aliment Pharmacol Ther, 2021, 53 (5): 577- 586.
doi: 10.1111/apt.16232 |
| 14 |
Sandborn WJ , Rebuck R , Wang Y , et al. Five-year efficacy and safety of ustekinumab treatment in Crohn's disease: The IMUNITI trial[J]. Clin Gastroenterol Hepatol, 2022, 20 (3): 578- 590.
doi: 10.1016/j.cgh.2021.02.025 |
| 15 |
Yao J Y , Zhang M , Wang W , et al. Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn's disease: A Chinese real-world study[J]. BMC Gastroenterol, 2021, 21 (1): 380.
doi: 10.1186/s12876-021-01946-8 |
| 16 |
Kucharzik T , Wilkens R , D'Agostino MA , et al. Early ultrasound response and progressive transmural remission after treatment with ustekinumab in Crohn's disease[J]. Clin Gastroenterol Hepatol, 2023, 21 (1): 153- 163.
doi: 10.1016/j.cgh.2022.05.055 |
| 17 |
Calabrese E , Rispo A , Zorzi F , et al. Ultrasonography tight control and monitoring in Crohn's disease during different biological therapies: A multicenter study[J]. Clin Gastroenterol Hepatol, 2022, 20 (4): e711- e722.
doi: 10.1016/j.cgh.2021.03.030 |
| 18 |
Feagan BG , Rubin DT , Danese S , et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists[J]. Clin Gastroenterol Hepatol, 2017, 15 (2): 229- 239.
doi: 10.1016/j.cgh.2016.08.044 |
| 19 |
Hanauer SB , Sandborn WJ , Feagan BG , et al. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn's disease[J]. J Crohns Colitis, 2020, 14 (1): 23- 32.
doi: 10.1093/ecco-jcc/jjz110 |
| 20 |
Ghosh S , Gensler LS , Yang Z , et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn's disease: An integrated analysis of phase Ⅱ/Ⅲ clinical development programs[J]. Drug Saf, 2019, 42 (6): 751- 768.
doi: 10.1007/s40264-019-00797-3 |
| [1] | Jun LI,Zhan-yue NIU,Yan XUE,Xue-ying SHI,Bo ZHANG,Yuan WANG. Kaposi sarcoma combined with severe ulcerative colitis: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 373-377. |
|
||