Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 756-762. doi: 10.19723/j.issn.1671-167X.2024.05.002
Previous Articles Next Articles
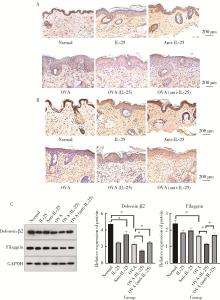
The role and its regulatory significance of interleukin-25 in ovalbumin induced atopic dermatitis of mice
Jiang JIN*( ), Xue CHEN, Yan ZHAO, Jun JIA, Jianzhong ZHANG
), Xue CHEN, Yan ZHAO, Jun JIA, Jianzhong ZHANG
- Department of Dermatology, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R758.2
| 1 |
Barbarot S , Auziere S , Gadkari A , et al. Epidemiology of atopic dermatitis in adults: Results from an international survey[J]. Allergy, 2018, 73 (6): 1284- 1293.
doi: 10.1111/all.13401 |
| 2 |
Aktar MK , Kido-Nakahara M , Furue M , et al. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis[J]. Allergy, 2015, 70 (7): 846- 854.
doi: 10.1111/all.12633 |
| 3 |
Fort MM , Cheung J , Yen D , et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo[J]. Immunity, 2001, 15 (6): 985- 995.
doi: 10.1016/S1074-7613(01)00243-6 |
| 4 |
Suto H , Nambu A , Morita H , et al. IL-25 enhances TH17 cell-mediated contact dermatitis by promoting IL-1β production by dermal dendritic cells[J]. J Allergy Clin Immunol, 2018, 142 (5): 1500- 1509.e10.
doi: 10.1016/j.jaci.2017.12.1007 |
| 5 |
Senra L , Mylonas A , Kavanagh RD , et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation[J]. J Invest Dermatol, 2019, 139 (8): 1732- 1742.e17.
doi: 10.1016/j.jid.2019.01.021 |
| 6 |
Tong X , Li B . A role of IL-25, a sibling of IL-17, in triggering psoriatic skin inflammation[J]. Sci China Life Sci, 2018, 61 (11): 1437- 1438.
doi: 10.1007/s11427-018-9330-x |
| 7 |
Hagner SL , Harb H , Zhao M , et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice[J]. Allergy, 2013, 68 (3): 322- 329.
doi: 10.1111/all.12094 |
| 8 |
Orciani M , Campanati A , Caffarini M , et al. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem[J]. Br J Dermatol, 2017, 176 (6): 1569- 1576.
doi: 10.1111/bjd.15078 |
| 9 |
Mashiko S , Mehta H , Bissonnette R , et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis[J]. J Dermatol Sci, 2017, 88 (2): 167- 174.
doi: 10.1016/j.jdermsci.2017.07.003 |
| 10 |
Furue M , Chiba T , Tsuji G , et al. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies[J]. Allergol Int, 2017, 66 (3): 398- 403.
doi: 10.1016/j.alit.2016.12.002 |
| 11 |
Kumar A , Rani L , Mhaske ST , et al. IL-3 receptor expression on activated human Th cells is regulated by IL-4, and IL-3 synergizes with IL-4 to enhance Th2 cell differentiation[J]. J Immunol, 2020, 204 (4): 819- 831.
doi: 10.4049/jimmunol.1801629 |
| 12 |
Ochiai S , Jagot F , Kyle RL , et al. Thymic stromal lymphopoietin drives the development of IL-13+ Th2 cells[J]. Proc Natl Acad Sci USA, 2018, 115 (5): 1033- 1038.
doi: 10.1073/pnas.1714348115 |
| 13 |
Coomes SM , Kannan Y , Pelly VS , et al. CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation[J]. Mucosal Immunol, 2017, 10 (1): 150- 161.
doi: 10.1038/mi.2016.47 |
| 14 |
Prout MS , Kyle RL , Ronchese F , et al. IL-4 is a key requirement for IL-4- and IL-4/IL-13-expressing CD4 Th2 subsets in lung and skin[J]. Front Immunol, 2018, 9, 1211.
doi: 10.3389/fimmu.2018.01211 |
| 15 |
Hönzke S , Wallmeyer L , Ostrowski A , et al. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and β-defensins in filaggrin-deficient skin equivalents[J]. J Invest Dermatol, 2016, 136 (3): 631- 639.
doi: 10.1016/j.jid.2015.11.007 |
| 16 |
Drislane C , Irvine AD . The role of filaggrin in atopic dermatitis and allergic disease[J]. Ann Allergy Asthma Immunol, 2020, 124 (1): 36- 43.
doi: 10.1016/j.anai.2019.10.008 |
| 17 |
Wallmeyer L , Dietert K , Sochorová M , et al. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents[J]. Sci Rep, 2017, 7 (1): 774.
doi: 10.1038/s41598-017-00670-2 |
| 18 | Christian L , Gerardus B , Marie-Anne V , et al. Novel microperfusion method confirms higher IL-17A and β-defensin-2 levels in psoriasis lesional skin compared to non-lesional skin[J]. J Dermatol Sci, 2016, 84 (1): 32- 33. |
| [1] | Shan-shan BAI,Si-yi MO,Xiao-xiang XU,Yun LIU,Qiu-fei XIE,Ye CAO. Characteristics of orofacial operant test for orofacial pain sensitivity caused by occlusal interference in rats [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 51-57. |
|
||