Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (2): 277-283. doi: 10.19723/j.issn.1671-167X.2025.02.009
Previous Articles Next Articles
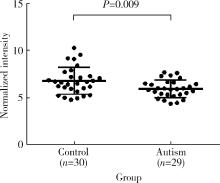
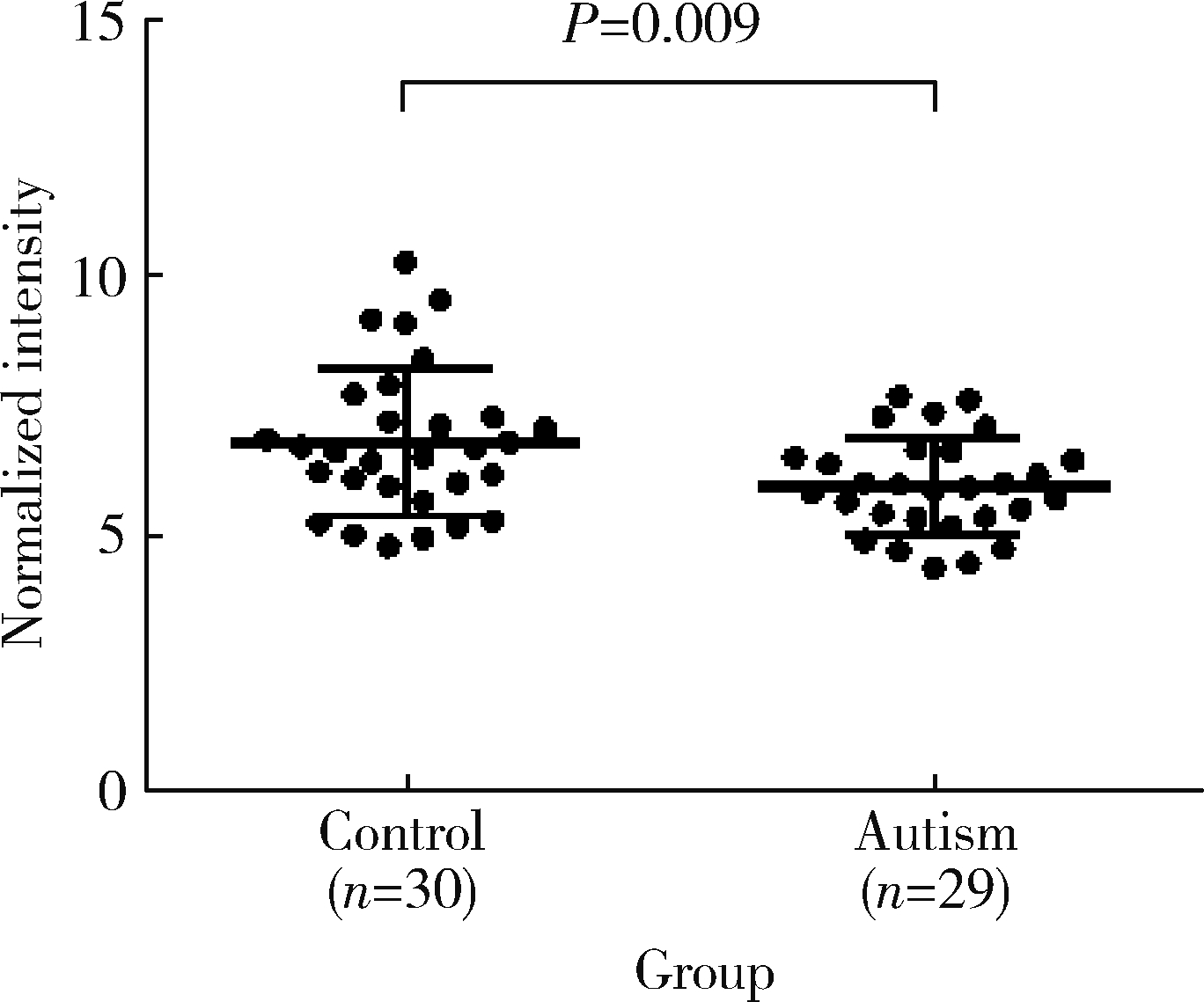
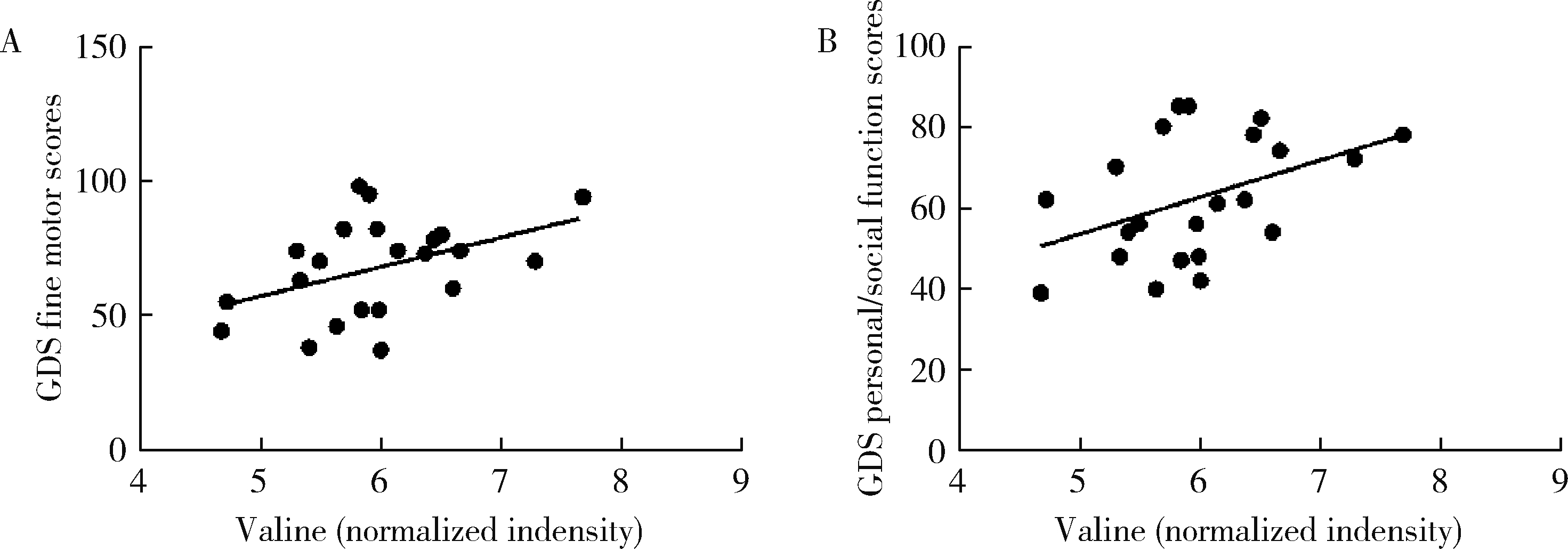
Change of plasma level of valine and its relationship with developmental quotient in children with autism
Xinjie XU1, Xiaoe CAI2, Fanchao MENG3, Bo LONG1, Xin YOU4, Rong ZHANG5,*( )
)
- 1. Medical Science Research Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2. Department of Rehabilitation Medicine, Beijing Haidian Hospital, Beijing 100080, China
3. The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing 100088, China
4. Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
5. Neuroscience Research Institute, Peking University; Department of Neurobiology, Peking University School of Basic Medical Sciences; Key Laboratory for Neuroscience, Ministry of Education/National Health and Family Planning Commission; Autism Research Center of Peking University Health Science Center, Beijing 100191, China
CLC Number:
- R749.94
| 1 | González MC , Vásquez M , Hernández-Chávez M . Autism spectrum disorder: Clinical diagnosis and ADOS test[J]. Rev Chil Pediatr, 2019, 90 (5): 485- 491. |
| 2 |
Maenner MJ , Shaw KA , Baio J , et al. Prevalence of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016[J]. MMWR Surveill Summ, 2020, 69 (4): 1- 12.
doi: 10.15585/mmwr.ss6904a1 |
| 3 |
Zhou H , Xu X , Yan W , et al. Prevalence of autism spectrum disorder in China: A nationwide multi-center population-based study among children aged 6 to 12 years[J]. Neurosci Bull, 2020, 36 (9): 961- 971.
doi: 10.1007/s12264-020-00530-6 |
| 4 |
樊越波, 揭晓锋, 邹小兵. 孤独症患病率回顾[J]. 中国儿童保健杂志, 2008, 16 (4): 439- 440.
doi: 10.3969/j.issn.1008-6579.2008.04.029 |
| 5 |
Croen LA , Zerbo O , Qian Y , et al. The health status of adults on the autism spectrum[J]. Autism, 2015, 19 (7): 814- 823.
doi: 10.1177/1362361315577517 |
| 6 | 赵刚, 韦明, 鄂颖梅, 等. 孤独症谱系障碍儿童饮食行为与家长喂养行为的相关研究[J]. 沈阳医学院学报, 2020, 22 (5): 428- 432. |
| 7 | Ghanizadeh A . Increased glutamate and homocysteine and decreased glutamine levels in autism: A review and strategies for future studies of amino acids in autism[J]. Dis Markers, 2013, 35 (5): 281- 286. |
| 8 | National Center for Biotechnology Information (2024). PubChem compound summary for CID 6287, valine[EB/OL]. [2021-03-08] https://pubchem.ncbi.nlm.nih.gov/compound/Valine. |
| 9 |
Maynard TM , Manzini MC . Balancing act: Maintaining amino acid levels in the autistic brain[J]. Neuron, 2017, 93 (3): 476- 479.
doi: 10.1016/j.neuron.2017.01.015 |
| 10 |
Tǎrlungeanu DC , Deliu E , Dotter CP , et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder[J]. Cell, 2016, 167 (6): 1481- 1494.e18.
doi: 10.1016/j.cell.2016.11.013 |
| 11 |
Smith AM , King JJ , West PR , et al. Amino acid dysregulation metabotypes: Potential biomarkers for diagnosis and individualized treatment for subtypes of autism spectrum disorder[J]. Biol Psychiatry, 2019, 85 (4): 345- 354.
doi: 10.1016/j.biopsych.2018.08.016 |
| 12 |
Sperringer JE , Addington A , Hutson SM . Branched-chain amino acids and brain metabolism[J]. Neurochem Res, 2017, 42 (6): 1697- 1709.
doi: 10.1007/s11064-017-2261-5 |
| 13 | 王强. Gesell发育量表对2岁以内孤独症谱系障碍(ASD)患儿的应用效果观察[J]. 世界最新医学信息文摘(连续型电子期刊), 2020, 20 (33): 61- 62. |
| 14 |
Larsson SC , Markus HS . Branched-chain amino acids and Alzheimer's disease: A Mendelian randomization analysis[J]. Sci Rep, 2017, 7 (1): 13604.
doi: 10.1038/s41598-017-12931-1 |
| 15 |
Bjerkenstedt L , Edman G , Hagenfeldt L , et al. Plasma amino acids in relation to cerebrospinal fluid monoamine metabolites in schizophrenic patients and healthy controls[J]. Br J Psychiatry, 1985, 147, 276- 282.
doi: 10.1192/bjp.147.3.276 |
| 16 | Tu WJ , Chen H , He J . Application of LC-MS/MS analysis of plasma amino acids profiles in children with autism[J]. J Clin Biochem Nutr, 2012, 51 (3): 248- 249. |
| 17 |
Arnold GL , Hyman SL , Mooney RA , et al. Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies[J]. J Autism Dev Disord, 2003, 33 (4): 449- 454.
doi: 10.1023/A:1025071014191 |
| 18 |
Witters P , Debbold E , Crivelly K , et al. Autism in patients with propionic academia[J]. Mol Genet Metab, 2016, 119 (4): 317- 321.
doi: 10.1016/j.ymgme.2016.10.009 |
| 19 | Bala KA , Dǒgan M , Mutluer T , et al. Plasma amino acid profile in autism spectrum disorder (ASD)[J]. Eur Rev Med Pharmacol Sci, 2016, 20 (5): 923- 929. |
| 20 |
Zou M , Li D , Wang L , et al. Identification of amino acid dys-regulation as a potential biomarker for autism spectrum disorder in China[J]. Neurotox Res, 2020, 38 (4): 992- 1000.
doi: 10.1007/s12640-020-00242-9 |
| 21 |
May T , Adesina I , McGillivray J , et al. Sex differences in neurodevelopmental disorders[J]. Curr Opin Neurol, 2019, 32 (4): 622- 626.
doi: 10.1097/WCO.0000000000000714 |
| 22 |
Lavelle A , Sokol H . Gut microbiota-derived metabolites as key actors in inflammatory bowel disease[J]. Nat Rev Gastroenterol Hepatol, 2020, 17 (4): 223- 237.
doi: 10.1038/s41575-019-0258-z |
| 23 |
Lussu M , Noto A , Masili A , et al. The urinary (1) H-NMR metabolomics profile of an Italian autistic children population and their unaffected siblings[J]. Autism Res, 2017, 10 (6): 1058- 1066.
doi: 10.1002/aur.1748 |
| 24 |
Li C , Shen K , Chu L , et al. Decreased levels of urinary free amino acids in children with autism spectrum disorder[J]. J Clin Neurosci, 2018, 54, 45- 49.
doi: 10.1016/j.jocn.2018.05.001 |
| 25 |
Evans C , Dunstan RH , Rothkirch T , et al. Altered amino acid excretion in children with autism[J]. Nutr Neurosci, 2008, 11 (1): 9- 17.
doi: 10.1179/147683008X301360 |
| 26 |
De Angelis M , Piccolo M , Vannini L , et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified[J]. PLoS One, 2013, 8 (10): e76993.
doi: 10.1371/journal.pone.0076993 |
| 27 |
Carunchio I , Curcio L , Pieri M , et al. Increased levels of p70S6 phosphorylation in the G93A mouse model of amyotrophic lateral sclerosis and in valine-exposed cortical neurons in culture[J]. Exp Neurol, 2010, 226 (1): 218- 230.
doi: 10.1016/j.expneurol.2010.08.033 |
| 28 |
Nave KA , Werner HB . Myelination of the nervous system: Mechanisms and functions[J]. Annu Rev Cell Dev Biol, 2014, 30, 503- 533.
doi: 10.1146/annurev-cellbio-100913-013101 |
| 29 |
Kakazu E , Kanno N , Ueno Y , et al. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells[J]. J Immunol, 2007, 179 (10): 7137- 7146.
doi: 10.4049/jimmunol.179.10.7137 |
| 30 | Shen L , Feng C , Zhang K , et al. Proteomics study of peripheral blood mononuclear cells (PBMCs) in autistic children[J]. Front Cell Neurosci, 2019, 13, 105. |
| 31 | Lungba RM , Khan S , Ajibawo-Aganbi U , et al. The role of the gut microbiota and the immune system in the development of autism[J]. Cureus, 2020, 12 (10): e11226. |
| 32 |
Meltzer A , Van de Water J . The role of the immune system in autism spectrum disorder[J]. Neuropsychopharmacology, 2017, 42 (1): 284- 298.
doi: 10.1038/npp.2016.158 |
| [1] | Ya-nan ZHAO,Hui-yun FAN,Xiang-yu WANG,Ya-nan LUO,Rong ZHANG,Xiao-ying ZHENG. Early death and causes of death of patients with autism spectrum disorders: A systematic review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 375-383. |
| [2] | JIAO Cui,WANG Jian-mei,KUANG Hai-xia,WU Zhi-hong,LIU Tao. Effects of CACNA1H gene knockout on autistic-like behaviors and the morphology of hippocampal neurons in mice [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 209-216. |
| [3] | SHI Hui-feng, ZHANG Jing-xu, ZHANG Rong, WANG Xiao-li. Prevalence of autism spectrum disorders in children aged 0-6 years in China: a meta-analysis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 798-806. |
|
||