1 资料与方法
1.1 研究对象
1.2 研究方法
1.2.1 基本信息及体格检查
1.2.2 孕前BMI计算及分级
1.2.3 孕期增重计算及分级
1.2.4 血液样本采集及检测
1.2.5 肝脏超声检查和脂肪性肝病诊断
1.2.6 CMRF异常的定义及诊断
1.2.7 MASLD的诊断与分组
1.2.8 不良妊娠结局的诊断
1.3 统计学分析
2 结果
表1 研究对象的基本资料及不良妊娠结局发生情况Table 1 Baseline characteristics and incidence of adverse pregnancy outcomes in participants |
| Variables | Control group(n=2 321) | MASLD group(n=302) | χ2/t | P |
| Maternal age/years | 31.20 ± 3.12 | 32.57 ± 3.64 | 6.23 | < 0.001 |
| < 35 | 2 075 (89.4) | 226 (74.8) | 51.31 | < 0.001 |
| ≥35 | 246 (10.6) | 76 (25.2) | ||
| Height/cm | 162.86±5.10 | 162.79±5.42 | 0.22 | 0.824 |
| Pre-pregnancy weight/kg | 53.98±5.61 | 74.54±11.88 | 29.64 | < 0.001 |
| Pre-pregnancy BMI/(kg/m2) | 20.34±1.75 | 28.07±3.85 | 34.46 | < 0.001 |
| Underweight | 329 (14.2) | 0 (0.0) | 2 294.87 | < 0.001 |
| Normal weight | 1 992 (85.8) | 34 (11.3) | ||
| Overweight | 0 (0.0) | 131 (43.4) | ||
| Obese | 0 (0.0) | 137 (45.4) | ||
| Weight before delivery/kg | 67.78±7.58 | 84.46±12.88 | 22.01 | < 0.001 |
| Gestational weight gain/kg | 13.81±4.92 | 9.92±6.41 | 10.16 | < 0.001 |
| Insufficient | 263 (11.3) | 79 (26.2) | 61.10 | < 0.001 |
| Adequate | 1 096 (47.3) | 93 (30.8) | ||
| Excessive | 959 (41.4) | 130 (43.0) | ||
| Systolic blood pressure/mmHg | 111.61±9.95 | 122.41±11.65 | 15.39 | < 0.001 |
| Diastolic blood pressure/mmHg | 66.02±7.91 | 73.97±9.26 | 14.27 | < 0.001 |
| Education level | ||||
| Below bachelor’s degree | 439 (18.9) | 86 (28.5) | 14.67 | < 0.001 |
| Bachelor’s degree or higher | 1 882 (81.1) | 216 (71.5) | ||
| Primiparous | 2 263 (97.5) | 295 (97.7) | < 0.001 | 1.000 |
| Chronic hypertension history | 0 (0.0) | 27 (8.9) | 200.98 | < 0.001 |
| Type 2 diabetes mellitus history | 0 (0.0) | 25 (8.3) | 185.32 | < 0.001 |
| Adverse pregnancy outcome | ||||
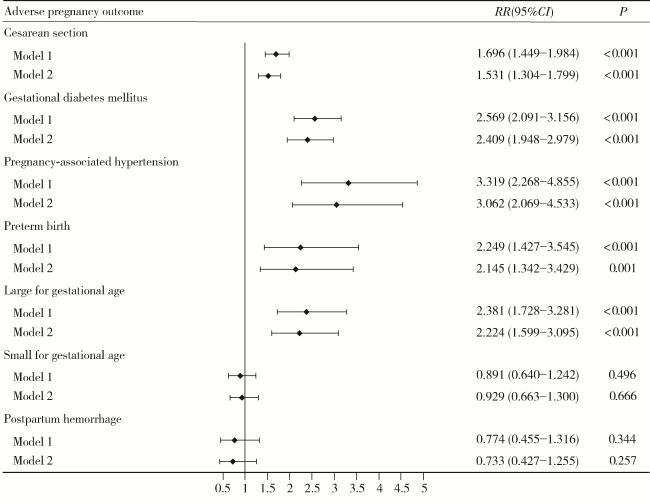
| Cesarean section | 861 (37.1) | 190 (62.9) | 73.11 | < 0.001 |
| Gestational diabetes mellitus | 362 (15.6) | 121 (40.1) | 104.88 | < 0.001 |
| Pregnancy-associated hypertension | 88 (3.8) | 38 (12.6) | 43.26 | < 0.001 |
| Preterm birth | 82 (3.5) | 24 (7.9) | 12.31 | < 0.001 |
| Large for gestational age | 158 (6.8) | 49 (16.2) | 31.26 | < 0.001 |
| Small for gestational age | 336 (14.5) | 39 (12.9) | 0.42 | 0.517 |
| Postpartum hemorrhage | 149 (6.4) | 15 (5.0) | 0.73 | 0.393 |
Categorical variables were presented as n (%), and compared using the Chi-square test; Continuous variables were presented as ${\bar x}$±s, and compared using the student’s t test. BMI, body mass index; MASLD, metabolic dysfunction-associated steatotic liver disease. |
表2 不同CMRF异常组的不良妊娠结局发生情况Table 2 Incidence of adverse pregnancy outcomes in different CMRF abnormalities groups |
| Adverse pregnancy outcome | BMI abnormal group (n=268) | Glucose abnormal group (n=46) | Blood pressure abnormal group (n=40) | HDL-C abnormal group (n=164) | TG abnormal group (n=83) | χ2 | P |
| Cesarean section | 166 (61.94) | 34 (73.91) | 26 (65.00) | 102 (62.20) | 48 (57.83) | 3.48 | 0.481 |
| Gestational diabetes mellitus | 107 (39.93) | 33 (71.74) | 19 (47.50) | 64 (39.02) | 37 (44.58) | 17.96 | 0.001 |
| Pregnancy associated hypertension | 35 (13.06) | 5 (10.87) | 4 (10.00) | 21 (12.80) | 10 (12.05) | 0.45 | 0.978 |
| Preterm birth | 21 (7.84) | 4 (8.70) | 5 (12.50) | 10 (6.10) | 8 (9.64) | 2.25 | 0.689 |
| Large for gestational age | 44 (16.42) | 11 (23.91) | 8 (20.00) | 22 (13.41) | 18 (21.69) | 4.63 | 0.328 |
| Small for gestational age | 34 (12.69) | 5 (10.87) | 7 (17.50) | 21 (12.80) | 11 (13.25) | 0.94 | 0.919 |
| Postpartum hemorrhage | 12 (4.48) | 4 (8.70) | 1 (2.50) | 9 (5.49) | 6 (7.23) | 2.66 | 0.617 |
The incidence was presented as n (%) and compared using the Chi-square test. BMI, body mass index; CMRF, cardiometabolic risk factor; HDL-C, high density lipoprotein cholesterol;TG, triglyceride. |
表3 不同CMRF异常MASLD与不良妊娠结局的相关性分析Table 3 Associations between different types of CMRF abnormalities in MASLD and the risk of adverse pregnancy outcomes |
| Adverse pregnancy outcome | Control group | BMI abnormal group | Glucose abnormal group | Blood pressure abnormal group | HDL-C abnormal group | TG abnormal group | |||||||||
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | ||||||
| Cesarean section | |||||||||||||||
| Model 1 | 1.000 | 1.670(1.414, 1.972) | < 0.001 | 1.992 (1.414, 2.807) | < 0.001 | 1.752 (1.186, 2.588) | 0.005 | 1.677(1.366, 2.059) | < 0.001 | 1.559 (1.166, 2.085) | 0.003 | ||||
| Model 2 | 1.000 | 1.498(1.264, 1.776) | < 0.001 | 1.617 (1.139, 2.298) | 0.007 | 1.509 (1.017, 2.240) | 0.041 | 1.518(1.232, 1.869) | < 0.001 | 1.363 (1.015, 1.830) | 0.040 | ||||
| Model 3 | 1.000 | 1.572(1.224, 2.018) | < 0.001 | 2.779 (0.796, 9.705) | 0.109 | 3.206 (0.714, 14.395) | 0.128 | 1.971(1.226, 3.170) | 0.005 | 1.371 (0.641, 2.934) | 0.416 | ||||
| Gestational diabetes mellitus | |||||||||||||||
| Model 1 | 1.000 | 2.560(2.063, 3.176) | < 0.001 | 4.600 (3.221, 6.569) | < 0.001 | 3.046 (1.920, 4.831) | < 0.001 | 2.502(1.918, 3.264) | < 0.001 | 2.85 8 (2.03 8, 4.009) | < 0.001 | ||||
| Model 2 | 1.000 | 2.411(1.930, 3.012) | < 0.001 | 3.825 (2.628, 5.567) | < 0.001 | 2.548 (1.591, 4.081) | < 0.001 | 2.284(1.741, 2.997) | < 0.001 | 2.506 (1.773, 3.544) | < 0.001 | ||||
| Model 3 | 1.000 | 2.308 (1.665, 3.198) | < 0.001 | 6.734 (1.934, 23.444) | 0.003 | 2.174 (0.267, 17.688) | 0.468 | 2.098(1.067, 4.126) | 0.032 | 2.862 (1.314, 6.235) | 0.008 | ||||
| Pregnancy-associated hypertension | |||||||||||||||
| Model 1 | 1.000 | 3.444(2.328, 5.096) | < 0.001 | 2.867 (1.164, 7.059) | 0.022 | 2.638 (0.968, 7.184) | 0.058 | 3.377(2.098, 5.436) | < 0.001 | 3.178 (1.652, 6.112) | < 0.001 | ||||
| Model 2 | 1.000 | 3.161(2.113, 4.728) | < 0.001 | 2.3 89 (0.944, 6.049) | 0.066 | 2.526 (0.913, 6.987) | 0.074 | 3.221(1.981, 5.239) | < 0.001 | 2.793 (1.427, 5.467) | 0.003 | ||||
| Model 3 | 1.000 | 3.288(1.859, 5.814) | < 0.001 | 6.097 (0.372, 100.030) | 0.205 | 15.649 (1.194, 205.006) | 0.036 | 3.764(1.172, 12.091) | 0.026 | 2.372 (0.319, 17.662) | 0.399 | ||||
| Preterm birth | |||||||||||||||
| Model 1 | 1.000 | 2.218(1.373, 3.582) | 0.001 | 2.461 (0.902, 6.715) | 0.079 | 3.538 (1.434, 8.727) | 0.006 | 1.726(0.895, 3.328) | 0.103 | 2.728 (1.320, 5.638) | 0.007 | ||||
| Model 2 | 1.000 | 2.185(1.333, 3.582) | 0.002 | 2.055 (0.728, 5.800) | 0.174 | 2.932 (1.161, 7.407) | 0.023 | 1.650(0.846, 3.217) | 0.142 | 2.408 (1.146, 5.062) | 0.020 | ||||
| Model 3 | 1.000 | 2.459(1.231, 4.913) | 0.011 | 1.239 (0.191, 8.030) | 0.823 | - | - | 1.333(0.182, 9.738) | 0.777 | 4.285 (1.031, 17.810) | 0.045 | ||||
| Large for gestational age | |||||||||||||||
| Model 1 | 1.000 | 2.410(1.725, 3.366) | < 0.001 | 3.510 (1.905, 6.467) | < 0.001 | 2.935 (1.443, 5.972) | 0.003 | 1.969(1.260, 3.076) | 0.003 | 3.183 (1.955, 5.183) | < 0.001 | ||||
| Model 2 | 1.000 | 2.249(1.595, 3.171) | < 0.001 | 3.270 (1.732, 6.174) | < 0.001 | 2.844 (1.382, 5.852) | 0.005 | 1.832(1.164, 2.884) | 0.009 | 3.019 (1.832, 4.976) | < 0.001 | ||||
| Model 3 | 1.000 | 2.266(1.388, 3.697) | 0.001 | 4.962 (0.485, 50.773) | 0.177 | - | - | 1.376(0.416, 4.549) | 0.601 | 3.223 (0.980, 10.593) | 0.054 | ||||
| Small for gestational age | |||||||||||||||
| Model 1 | 1.000 | 0.876(0.615, 1.246) | 0.460 | 0.750 (0.310, 1.814) | 0.523 | 1.208 (0.571, 2.553) | 0.621 | 0.884(0.569, 1.373) | 0.583 | 0.915 (0.502, 1.668) | 0.771 | ||||
| Model 2 | 1.000 | 0.916(0.641, 1.310) | 0.631 | 0.785 (0.322, 1.916) | 0.596 | 1.227 (0.577, 2.606) | 0.595 | 0.909(0.583, 1.419) | 0.676 | 0.949 (0.519, 1.738) | 0.867 | ||||
| Model 3 | 1.000 | 0.925(0.536, 1.594) | 0.778 | 4.515 (0.564, 36.128) | 0.155 | - | - | 1.265(0.467, 3.432) | 0.644 | 1.132 (0.275, 4.656) | 0.863 | ||||
| Postpartum hemorrhage | |||||||||||||||
| Model 1 | 1.000 | 0.697(0.387, 1.256) | 0.230 | 1.355 (0.502, 3.657) | 0.549 | 0.389 (0.054, 2.783) | 0.347 | 0.855(0.436, 1.675) | 0.648 | 1.126 (0.498, 2.547) | 0.776 | ||||
| Model 2 | 1.000 | 0.668(0.369, 1.212) | 0.185 | 1.205 (0.439, 3.313) | 0.717 | 0.349 (0.049, 2.508) | 0.296 | 0.806(0.409, 1.589) | 0.533 | 1.014 (0.445, 2.313) | 0.974 | ||||
| Model 3 | 1.000 | 0.487(0.167, 1.420) | 0.188 | 2.519 (0.134, 47.306) | 0.537 | - | - | 0.503(0.065, 3.890) | 0.510 | 2.517 (0.628, 10.086) | 0. 192 | ||||
Model 1 was unadjusted; Model 2 was adjusted for age, education level, and gestational weight gain; Model 3 was adjusted for age, education level, gestational weight gain, BMI abnormality, glucose abnormality, blood pressure abnormality, HDL-C abnormality, and TG abnormality. However, when any of these variables was the dependent variable, it was not adjusted as a covariate. BMI, body mass index; CMRF, cardiometabolic risk factor; HDL-C, high density lipoprotein cholesterol; MASLD, metabolic dysfunction-associated steatotic liver disease; TG, triglyceride. -, indicates unstable risk estimation results. |
表4 不同CMRF异常程度组的不良妊娠结局Table 4 Incidence of adverse pregnancy outcomes in groups with different degrees of CMRF abnormalities |
| Adverse pregnancy outcome | Control group (n=2 321) | Mild CMRF abnormality group (n=104) | Moderate CMRF abnormality group (n=125) | Severe CMRF abnormality group (n=73) | χ2 | P |
| Cesarean section | 861 (37.1) | 65 (62.50) | 78 (62.40) | 47 (64.38) | 74.26 | < 0.001 |
| Gestational diabetes mellitus | 362 (15.6) | 36 (34.62) | 48 (38.40) | 37 (50.68) | 114.27 | < 0.001 |
| Pregnancy-associated hypertension | 88 (3.8) | 10 (9.62) | 19 (15.20) | 9 (12.33) | 49.05 | < 0.001 |
| Preterm birth | 82 (3.5) | 7 (6.73) | 11 (8.80) | 6 (8.22) | 14.07 | 0.003 |
| Large for gestational age | 158 (6.8) | 19 (18.27) | 13 (10.40) | 17 (23.29) | 43.97 | < 0.001 |
| Small for gestational age | 336 (14.5) | 11 (10.58) | 19 (15.20) | 9 (12.33) | 1.56 | 0.669 |
| Postpartum hemorrhage | 149 (6.4) | 3 (2.88) | 8 (6.40) | 4 (5.48) | 2.20 | 0.532 |
The incidence was presented as n (%) and compared using the Chi-square test. CMRF, cardiometabolic risk factor. |
表5 CMRF异常程度变化与不良妊娠结局的相关性分析Table 5 Associations between change in degree of CMRF abnormality and the risk of adverse pregnancy outcomes |
| Adverse pregnancy outcome | RR (95%CI) | P |
| Cesarean section | ||
| Model 1 | 1.258 (1.169, 1.354) | < 0.001 |
| Model 2 | 1.199 (1.112, 1.292) | < 0.001 |
| Gestational diabetes mellitus | ||
| Model 1 | 1.529 (1.396, 1.675) | < 0.001 |
| Model 2 | 1.478 (1.345, 1.624) | < 0.001 |
| Pregnancy-associated hypertension | ||
| Model 1 | 1.672 (1.416, 1.974) | < 0.001 |
| Model 2 | 1.626 (1.367, 1.934) | < 0.001 |
| Preterm birth | ||
| Model 1 | 1.432 (1.166, 1.758) | < 0.001 |
| Model 2 | 1.384 (1.120, 1.710) | 0.003 |
| Large for gestational age | ||
| Model 1 | 1.465 (1.268, 1.692) | < 0.001 |
| Model 2 | 1.422 (1.224, 1.650) | < 0.001 |
| Small for gestational age | ||
| Model 1 | 0.962 (0.820, 1.129) | 0.639 |
| Model 2 | 0.981 (0.834, 1.153) | 0.812 |
| Postpartum hemorrhage | ||
| Model 1 | 0.929 (0.723, 1.194) | 0.567 |
| Model 2 | 0.905 (0.702, 1.167) | 0.443 |
Model 1 was unadjusted; Model 2 was adjusted for age, education level, and gestational weight gain. CMRF, cardiometabolic risk factor. |