Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (3): 487-495. doi: 10.19723/j.issn.1671-167X.2025.03.012
Previous Articles Next Articles
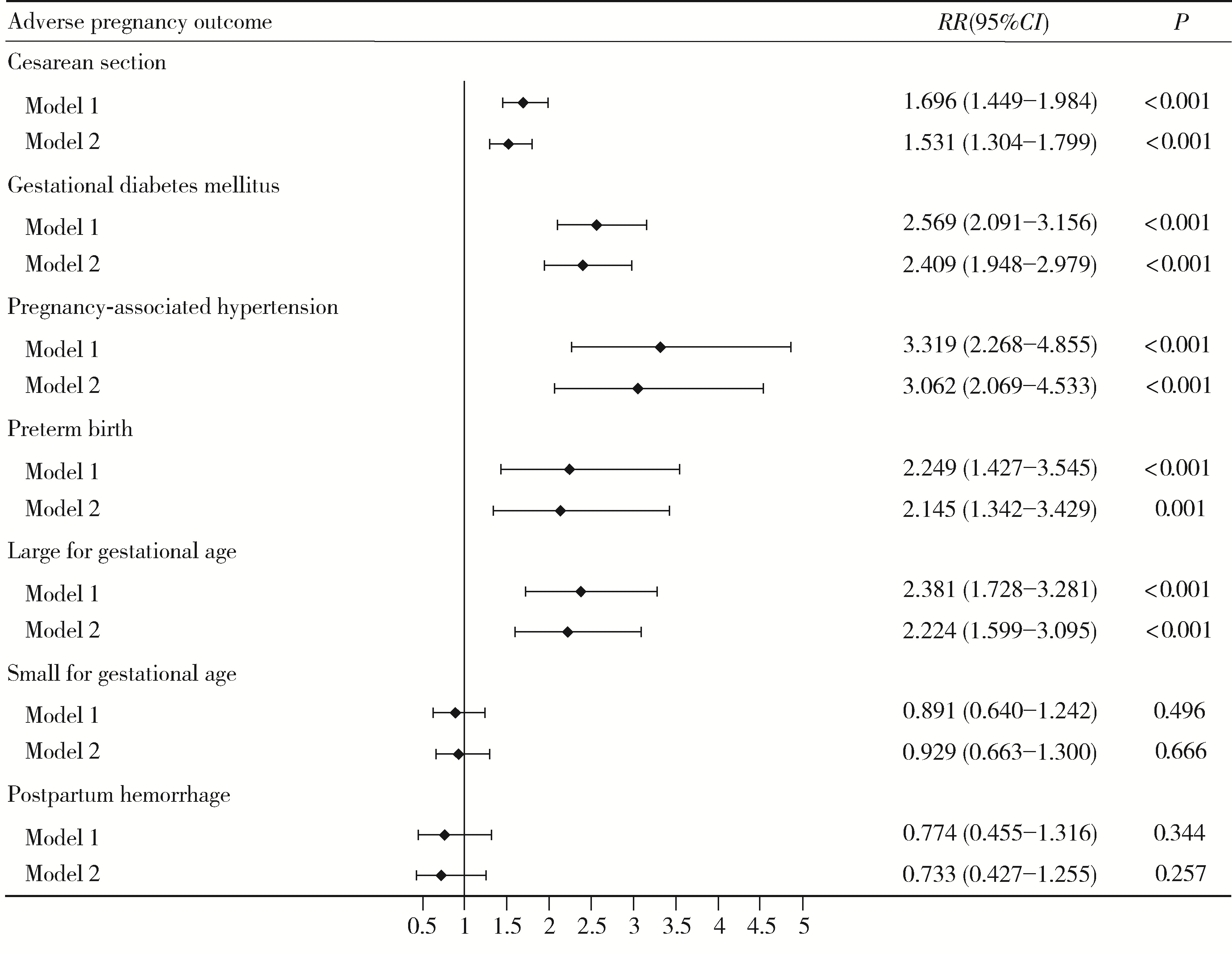
Associations of metabolic dysfunction-associated steatotic liver disease and cardiometabolic risk factor abnormalities with adverse pregnancy outcomes
Shuhan YANG1, Yixin LI2, Haoliang CUI3, Youxin WANG1, Yuying WU1, Mingyue WANG1, Yifan YANG1, Nur Enkar1, Lei YANG4,*( ), Hui WANG1,*(
), Hui WANG1,*( )
)
- 1. Department of Maternal and Child Health, School of Public Health, Peking University, Beijing 100191, China
2. Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing 100191, China
3. Department of Global Health, School of Public Health, Peking University, Beijing 100191, China
4. Department of Obstetrics and Gynecology, Beijing Friendship Hospital, Capital University of Medical Sciences, Beijing 100050, China
CLC Number:
- R714.25
| 1 |
doi: 10.1111/jgh.16554 |
| 2 |
doi: 10.1016/j.jhep.2020.03.049 |
| 3 |
窦紫岩, 钱文红, 孔邻润, 等. 非酒精性脂肪性肝病检出率现状及其影响因素: 基于北京市32万人群数据[J]. 中国全科医学, 2024, 27 (2): 144-149, 155.
|
| 4 |
doi: 10.1056/NEJMra1011035 |
| 5 |
doi: 10.1161/HYPERTENSIONAHA.115.06667 |
| 6 |
doi: 10.1016/j.jhep.2016.05.013 |
| 7 |
中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 中华肝脏病杂志, 2024, 32 (5): 418- 434.
|
| 8 |
doi: 10.1097/HEP.0000000000000520 |
| 9 |
doi: 10.1097/MEG.0000000000002802 |
| 10 |
|
| 11 |
|
| 12 |
doi: 10.1038/s41440-023-01354-3 |
| 13 |
中华人民共和国国家卫生和计划生育委员会. 成人体重判定[S]. 北京: 中国标准出版社, 2013.
|
| 14 |
中华人民共和国国家卫生健康委员会. 妊娠期妇女体重增长推荐值标准[S]. 北京: 中国标准出版社, 2022.
|
| 15 |
中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018更新版)[J]. 中华肝脏病杂志, 2018, 26 (3): 195- 203.
|
| 16 |
doi: 10.2337/dc09-1848 |
| 17 |
Hypertension in pregnancy: Executive summary [J]. Obstet Gynecol, 2013, 122(5): 1122-1131.
|
| 18 |
doi: 10.1097/AOG.0000000000001711 |
| 19 |
doi: 10.1111/j.1651-2227.1996.tb14164.x |
| 20 |
中华人民共和国国家卫生健康委员会. 不同胎龄新生儿出生时生长评价标准[S]. 北京: 中国标准出版社, 2022.
|
| 21 |
谢幸, 孔北华, 段涛, 等. 妇产科学[M]. 9版 北京: 人民卫生出版社, 2018: 204.
|
| 22 |
doi: 10.1210/clinem/dgac567 |
| 23 |
|
| 24 |
doi: 10.1186/s12884-024-06556-2 |
| 25 |
doi: 10.1016/j.wombi.2019.06.011 |
| 26 |
|
| 27 |
doi: 10.1038/ncomms13496 |
| 28 |
doi: 10.3390/ijms24087465 |
| 29 |
doi: 10.1016/j.jacc.2010.05.034 |
| 30 |
doi: 10.1373/clinchem.2019.303990 |
| 31 |
doi: 10.1007/s00467-020-04579-3 |
| 32 |
doi: 10.1038/s41366-023-01299-0 |
| 33 |
doi: 10.3390/ijerph191710846 |
| [1] | Wenqiong WANG, Yuke HOU, Chun LI, Xuewu ZHANG. Predictors of adverse pregnancy outcomes in patients with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 599-603. |
| [2] | Wen BIAN, Wenjun ZHOU, Tianchen WU, Peijing ZHU, Yinuo CHEN, Pengbo YUAN, Xueju WANG, Ying WANG, Yuan WEI, Yangyu ZHAO. Perinatal outcomes of single intrauterine fetal demise in monochorionic diamniotic twins [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 592-598. |
| [3] | Huichao MA,Jun LI,Yongqing WANG. Clinical characteristics of pregnancy complicated with inflammatory bowel disease [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 260-266. |
| [4] | Fang-ning YOU,Liang LUO,Xiang-jun LIU,Xue-wu ZHANG,Chun LI. Analysis of pregnancy outcomes, disease progression, and risk factors in patients with undifferentiated connective tissue disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1045-1052. |
| [5] | Xi-ya SUN,Yi-lu CHEN,Lin ZENG,Li-ying YAN,Jie QIAO,Rong LI,Xu ZHI. Correlation analysis of vitamin D level and anti-Müllerian hormone in infertile female and the role in predicting pregnancy outcome [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 167-173. |
| [6] | Wei-qian CHEN,Xiao-na DAI,Ye YU,Qin WANG,Jun-yu LIANG,Yi-ni KE,Cai-hong YI,Jin LIN. Analysis of clinical features and prognosis in patients with primary Sjögren’s syndrome and autoimmune liver disease [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 886-891. |
| [7] | CHEN Xiao-mei, LI Fu-qiang, YAN Su, WU Xiao-cui, TANG Cui-lan. Nicotine alleviates the liver inflammation of non-alcoholic steatohepatitis induced by high-fat and high-fructose in mice [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 777-782. |
| [8] | ZHENG Xiao-Juan, DENG Xiao-Li, LIU Xiang-Yuan. Pregnancy outcome in 54 patients with antiphospholipid syndrome:a retrospective clinical study [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 323-328. |
| [9] | REN Yun, YANG Shuo, YANG Rui, LI Rong, CHEN Xin-na, WANG Hai-yan, MA Cai-hong, LIU Ping, QIAO Jie. Comparison of gonadotropin releasing hormone agonist long protocol and gonadotropin releasing hormone antagonist protocol in infertile women [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 877-881. |
|
||