1 病例资料
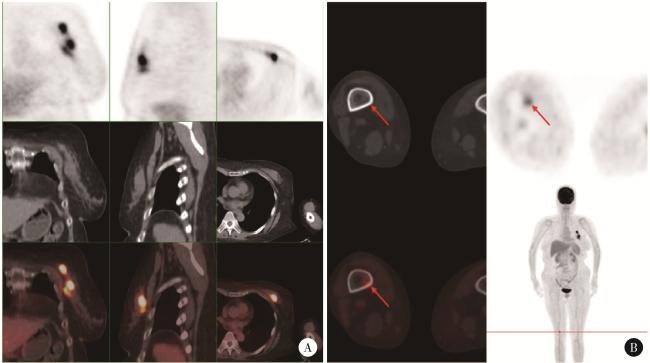
图1 患者PET/CT检查结果(2023年10月)Figure 1 PET/CT images of the patient (October 2023) A, left breast, multiple foci of increased flurodeoxyglucose (FDG) metabolism with a maximum standardized uptake value (SUVmax) of 10.1; B, right femur (distal), increased FDG metabolism with an SUVmax of 4.6. |
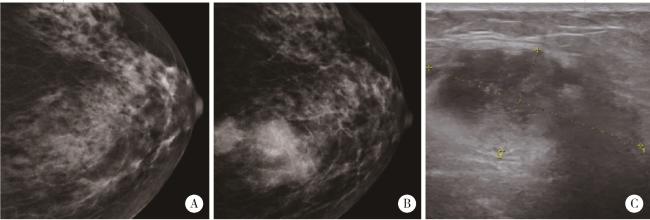
图2 左乳腺肿块钼靶X线检查Figure 2 Left breast mass examination A, mammogram (October 2021), multiple flaky or slightly high-density shadows are observed, with no apparent tumor identified; B, mammogram (October 2023), multiple circular and slightly high-density shadows are noted in the upper quadrant, approximately 8.5 cm from the nipple. The most extensive lesion measures approximately 3.5 cm×2.6 cm, with slightly blurred margins (Assessment: BI-RADS 4C); C, breast ultrasound (October 2023), a hypoechoic mass measuring 4.0 cm×1.9 cm is identified, exhibiting an irregular shape, indistinct margins, and no significant posterior acoustic changes (Assessment: BI-RADS 4B). |