Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (1): 21-27. doi: 10.19723/j.issn.1671-167X.2019.01.005
Previous Articles Next Articles
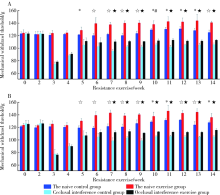
Effect of long-term resistance exercise on masseter muscle mechanical hyperalgesia in rats
Shu-dong YAN,Guang-ju YANG( ),Si-yi MO,Yun LIU,Qiu-fei XIE(
),Si-yi MO,Yun LIU,Qiu-fei XIE( )
)
- Department of Prosthodontics,Center for Oral and Jaw Functional Diagnosis, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology,Beijing 100081, China
CLC Number:
- R78
| [1] |
Soyannwo OA . Improved neuropathic pain treatment in developing countries-a critical review of WHO essential list[J]. Pain, 2015,156(5):763-764.
doi: 10.1097/j.pain.0000000000000140 pmid: 25719619 |
| [2] |
Cavalieri TA . Management of pain in older adults[J]. J Am Osteopath Assoc, 2005,105(Suppl 3):12-19.
doi: 10.1016/B978-0-323-08340-9.00034-7 pmid: 18154193 |
| [3] |
Dworkin RH, O’Connor AB, Audette J , et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update[J]. Mayo Clin Proc, 2010,85(3):S3-S14.
doi: 10.4065/mcp.2009.0649 pmid: 20194146 |
| [4] |
O’Connor AB, Dworkin RH . Treatment of neuropathic pain: an overview of recent guidelines[J]. Am J Med, 2009,122(Suppl 10):S22-S32
doi: 10.1016/j.amjmed.2009.04.007 pmid: 19801049 |
| [5] |
Latham N, Liu C . Strength training in older adults: the benefits for osteoarthritis[J]. Clin Geriatr Med, 2010,26(3):445-459.
doi: 10.1016/j.cger.2010.03.006 pmid: 3606891 |
| [6] |
Jansen M J, Viechtbauer W, Lenssen AF , et al. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review[J]. J Physiother, 2011,57(1):11-20.
doi: 10.1016/S1836-9553(11)70002-9 pmid: 0034625 |
| [7] |
Strasser B, Leeb G, Strehblow C , et al. The effects of strength and endurance training in patients with rheumatoid arthritis[J]. Clin Rheumatol, 2011,30(5):623-632.
doi: 10.1007/s10067-010-1584-2 pmid: 20931346 |
| [8] |
Harts CC, Helmhout PH, de Bie RA , et al. A high-intensity lumbar extensor strengthening program is little better than a low-intensity program or a waiting list control group for chronic low back pain: a randomised clinical trial[J]. Aust J Physiother, 2008,54(1):23-31.
doi: 10.1016/S0004-9514(08)70062-X pmid: 18298356 |
| [9] |
Kami K, Tajima F, Senba E . Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain[J]. Anat Sci Int, 2017,92(1):79-90.
doi: 10.1007/s12565-016-0360-z |
| [10] |
Cao Y, Xie QF, Li K , et al. Experimental occlusal interference induces long-term masticatory muscle hyperalgesia in rats[J]. Pain, 2009,144(3):287-293.
doi: 10.1016/j.pain.2009.04.029 pmid: 19473767 |
| [11] |
Cao Y, Li K, Fu KY , et al. Central sensitization and MAPKs are involved in occlusal interference-Induced facial pain in rats[J]. J Pain, 2013,14(8):793-807.
doi: 10.1016/j.jpain.2013.02.005 pmid: 3735867 |
| [12] |
李雪姣, 曹烨, 谢秋菲 , 等. 牙合干扰致大鼠咀嚼肌机械痛觉过敏的特点研究[J]. 中华口腔医学杂志, 2014,49(10):596-599.
doi: 10.3760/cma.j.issn.1002-0098.2014.10.006 |
| [13] |
Klitgaard H . A model for quantitative strength training of hindlimb muscles of the rat[J]. J Appl Physiol, 1988,64(4):1740-1745.
doi: 10.1055/s-2007-1025001 pmid: 3379005 |
| [14] |
Norenberg KM, Fitts RH . Contractile responses of the rat gastrocnemius and soleus muscles to isotonic resistance exercise[J]. J Appl Physiol, 2004,97(6):2322-2332.
doi: 10.1152/japplphysiol.00955.2003 pmid: 15322071 |
| [15] |
De LA, Pierno S, Liantonio A , et al. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1[J]. J Pharmacol Exp Ther, 2003,304(1):453-463.
doi: 10.1124/jpet.102.041343 pmid: 12490622 |
| [16] |
Jr HT, Farrar RP . Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat[J]. Can J Appl Physiol, 2004,29(1):16-31.
doi: 10.1139/h04-002 pmid: 15001801 |
| [17] |
Alves JP, Nunes RB, Stefani GP , et al. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure[J]. PLoS One, 2014,9(10):e110317.
doi: 10.1371/journal.pone.0110317 pmid: 4207701 |
| [18] | Lee S, Farrar RP . Resistance training induces muscle-specific changes in muscle mass and function in rat[J]. J Exerc Physiol Online, 2003,6(2):80-87. |
| [19] |
Safarzade A, Talebigarakani E . Short term resistance training enhanced plasma apoA-I and FABP4 levels in streptozotocin-induced diabetic rats[J]. J Diabetes Metab Disord, 2014,13(1):41.
doi: 10.1186/2251-6581-13-41 pmid: 24593955 |
| [20] |
Gerrits MM, Van Oppen P, Leone SS , et al. Pain, not chronic disease, is associated with the recurrence of depressive and anxiety disorders[J]. BMC psychiatry, 2014,14(1):187-198.
doi: 10.1186/1471-244X-14-187 pmid: 24965597 |
| [21] |
Yazdi M, Yilmaz Z, Renton T , et al. Psychological morbidity in chronic orofacial pain and headaches[J]. Oral Surgery, 2012,5(4):173-181.
doi: 10.1111/ors.12000 |
| [22] |
Weisberg JN, Boatwright BA . Mood, anxiety and personality traits and states in chronic pain[J]. Pain, 2007,133(1/2/3):1-2.
doi: 10.1016/j.pain.2007.10.005 pmid: 17967507 |
| [23] |
Tamaki T, Uchiyama S, Nakano S . A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats[J]. Med Sci Sports Exerc, 1992,24(8):881-886.
doi: 10.1249/00005768-199208000-00009 pmid: 1406173 |
| [24] |
Legerlotz K, Schjerling P, Langberg H , et al. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats[J]. J Appl Physiol, 2007,102(2):564-572.
doi: 10.1152/japplphysiol.00767.2006 pmid: 17038489 |
| [25] |
刘存瑞, 徐啸翔, 曹烨 , 等. 咬合干扰时间因素对大鼠咀嚼肌机械痛觉敏感的影响[J]. 北京大学学报(医学版), 2016,48(1):51-56.
doi: 10.3969/j.issn.1671-167X.2016.01.009 |
| [26] |
曹烨, 李锴, 傅开元 , 等. 咬合干扰致大鼠咬肌组织蛋白基因产物及P物质表达变化[J]. 北京大学学报(医学版), 2010,42(1):50-55.
doi: 10.3969/j.issn.1671-167X.2010.01.012 |
| [27] |
Merrill RL . Central mechanisms of orofacial pain[J]. Dent Clin North Am, 2007,51(1):45-59.
doi: 10.1016/j.cden.2006.09.010 pmid: 17185059 |
| [28] | 刘念, 臧凯凯, 张玉秋 . 外周神经损伤小鼠不同脊髓节段小胶质细胞和星形胶质细胞的激活状态[J]. 生理学报, 2015,67(6):571-582. |
| [29] |
高永静, 纪如荣 . 星形胶质细胞调节慢性疼痛的分子机制[J]. 中国疼痛医学杂志, 2013,19(9):545-552.
doi: 10.3969/j.issn.1006-9852.2013.09.009 |
| [30] |
Piao ZG, Cho IH, Park CK , et al. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury[J]. Pain, 2006,121(3):219-231.
doi: 10.1016/j.pain.2005.12.023 pmid: 16495005 |
| [31] |
Kuphal KE, Fibuch EE, Taylor BK . Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents[J]. J Pain, 2007,8(12):989-997.
doi: 10.1016/j.jpain.2007.08.001 pmid: 17890162 |
| [32] |
Shen J, Fox LE, Cheng J . Swim therapy reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction nerve injury in rats[J]. Pain Med, 2013,14(4):516-525.
doi: 10.1111/pme.12057 pmid: 3625453 |
| [33] |
Koltyn KF, Brellenthin AG, Cook DB , et al. Mechanisms of exercise-induced hypoalgesia.[J]. J Pain, 2014,15(12):1294-1304.
doi: 10.1016/j.jpain.2014.09.006 pmid: 4302052 |
| [34] |
Chen YW, Li YT, Chen YC , et al. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve.[J]. Anesth Analg, 2012,114(6):1330-1337.
doi: 10.1213/ANE.0b013e31824c4ed4 pmid: 22415536 |
| [35] |
Bobinski F, Martins DF, Bratti T , et al. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice.[J]. Neuroscience, 2011,194:337-348.
doi: 10.1016/j.neuroscience.2011.07.075 pmid: 21864654 |
| [36] | Katsuya K, Taguchi MS, Fumihiro T , et al. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain [J/OL]. Mol Pain, 2016, 12[2018-10-01]. |
| [37] |
Millan MJ . Descending control of pain[J]. Prog Neurobiol, 2002,66(6):355-474.
doi: 10.1016/S0301-0082(02)00009-6 |
| [38] |
Bobinski F, Taa F, Córdova MM , et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice[J]. Pain, 2015,156(12):2595-2606.
doi: 10.1097/j.pain.0000000000000372 pmid: 26447701 |
| [1] | FAN Ying-ying,LIU Yun,CAO Ye,XIE Qiu-fei. Hippocampus is involved in 17β-estradiol exacerbating experimental occlusal inter-ference-induced chronic masseter hyperalgesia in ovariectomized rats [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 40-47. |
|
||