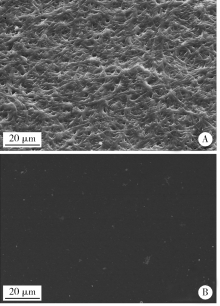
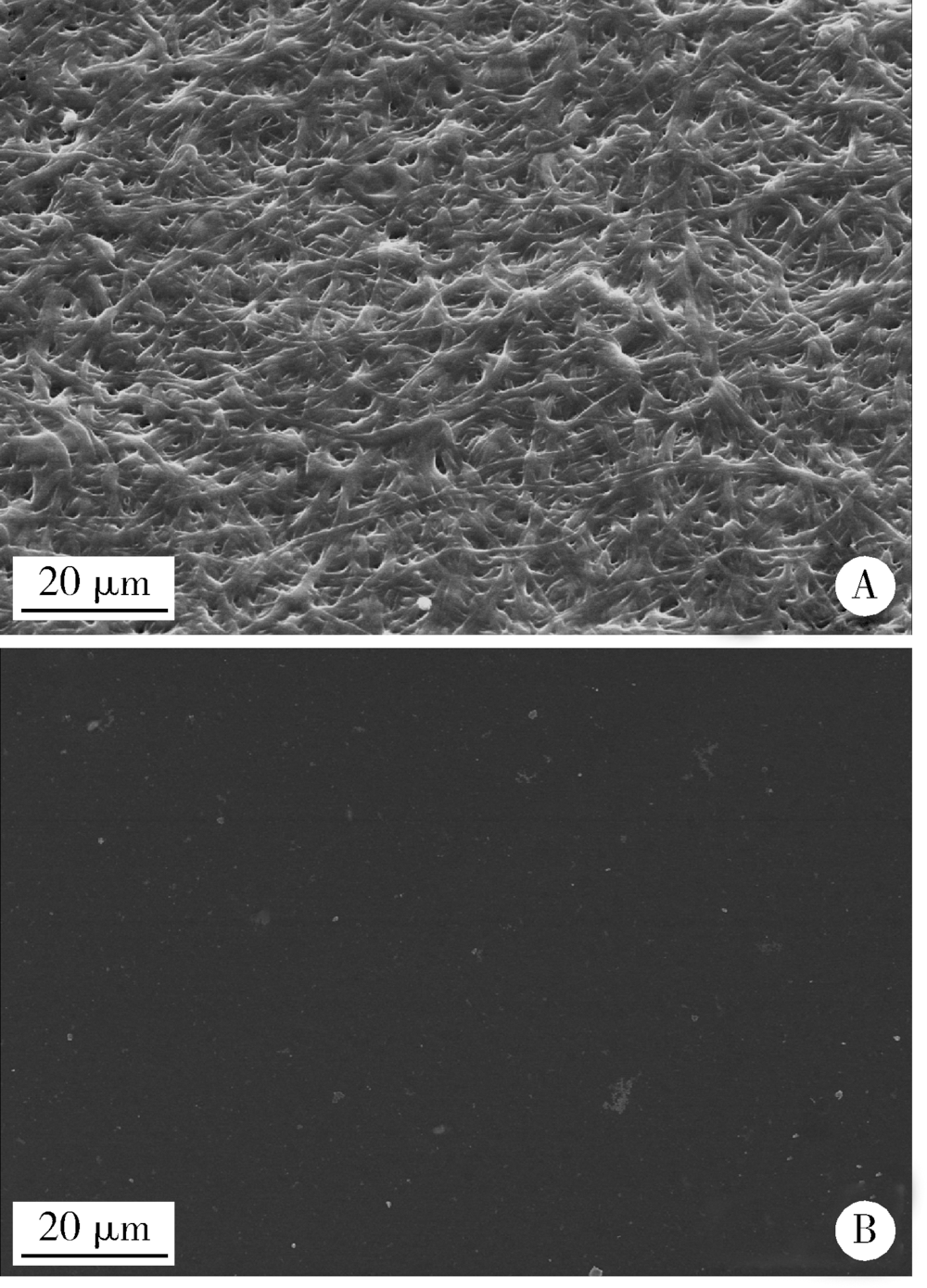
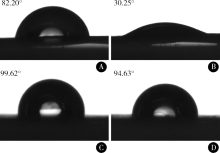
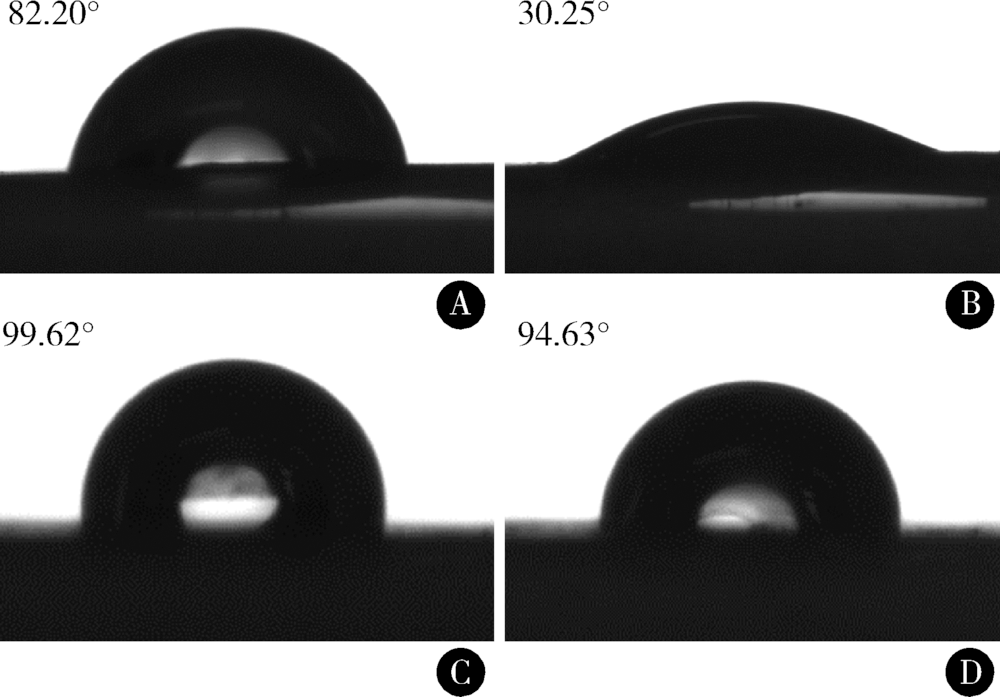
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (1): 28-34. doi: 10.19723/j.issn.1671-167X.2019.01.006
Previous Articles Next Articles
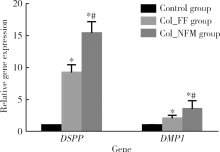
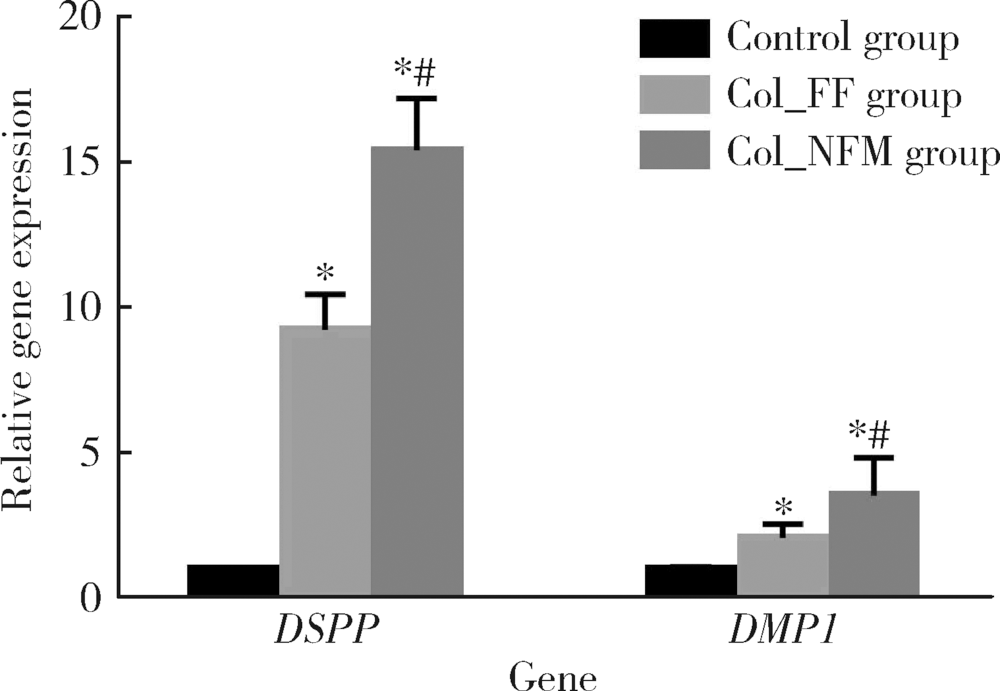
Effects of electrospun collagen nanofibrous matrix on the biological behavior of human dental pulp cells
Qian-li ZHANG1,Chong-yang YUAN1,Li LIU2,Shi-peng WEN2,△( ),Xiao-yan WANG1,△(
),Xiao-yan WANG1,△( )
)
- 1. Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Beijing Engineering Research Centre of Advanced Elastomers, Beijing University of Chemical Technology, Beijing 100029, China
CLC Number:
- R781.3
| [1] |
Lysaght MJ, Reyes J . The growth of tissue engineering[J]. Tissue Eng, 2001,7(5):485-493.
doi: 10.1089/107632701753213110 pmid: 11694183 |
| [2] |
Malhotra N, Kundabala M, Acharya S. Current strategies and applications of tissue engineering in dentistry: a review part 1 [J]. Dent Update, 2009, 36(9): 577- 579, 581-582.
pmid: 20099610 |
| [3] |
Wiesmann HP, Meyer U, Plate U , et al. Aspects of collagen mineralization in hard tissue formation[J]. Int Rev Cytol, 2005,242:121-156.
doi: 10.1016/S0074-7696(04)42003-8 pmid: 15598468 |
| [4] |
Sumita Y, Honda MJ, Ohara T , et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering[J]. Biomaterials, 2006,27(17):3238-3248.
doi: 10.1016/j.biomaterials.2006.01.055 pmid: 16504285 |
| [5] |
Prescott RS, Alsanea R, Fayad MI , et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice[J]. J Endod, 2008,34(4):421-426.
doi: 10.1016/j.joen.2008.02.005 pmid: 18358888 |
| [6] |
Kim NR, Lee DH, Chung PH , et al. Distinct differentiation pro-perties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2009,108(5):94-100.
doi: 10.1016/j.tripleo.2009.07.031 pmid: 19836718 |
| [7] |
Strom SC, Michalopoulos G . Collagen as a substrate for cell growth and differentiation[J]. Methods Enzymol, 1982,82(Pt A):544-555.
doi: 10.1016/0076-6879(82)82086-7 |
| [8] |
Grinnell F, Bennett MH . Ultrastructural studies of cell: collagen interactions[J]. Methods Enzymol, 1982,82(Pt A):535-544.
doi: 10.1016/0076-6879(82)82085-5 |
| [9] |
Elsdale T, Bard J . Collagen substrata for studies on cell behavior[J]. J Cell Biol, 1972,54(3):626-637.
doi: 10.1083/jcb.54.3.626 |
| [10] |
Woo KM, Chen VJ, Ma PX . Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment[J]. J Biomed Mater Res A, 2003,67(2):531-537.
doi: 10.1002/jbm.a.10098 pmid: 14566795 |
| [11] |
Wang J, Ma H, Jin X , et al. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells[J]. Biomaterials, 2011,32(31):7822-7830.
doi: 10.1016/j.biomaterials.2011.04.034 pmid: 3159766 |
| [12] |
Kuang R, Zhang Z, Jin X , et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells[J]. Adv Healthc Mater, 2015,4(13):1993-2000.
doi: 10.1002/adhm.201500308 pmid: 26138254 |
| [13] |
Kwon YS, Lee SH, Hwang YC , et al. Behaviour of human dental pulp cells cultured in a collagen hydrogel scaffold cross-linked with cinnamaldehyde[J]. Int Endod J,2017,50(1):58-66.
doi: 10.1111/iej.12592 pmid: 26650820 |
| [14] |
Coyac BR, Chicatun F, Hoac B , et al. Mineralization of dense collagen hydrogel scaffolds by human pulp cells[J]. J Dent Res, 2013,92(7):648-654.
doi: 10.1177/0022034513488599 pmid: 23632809 |
| [15] |
Pan S, Dangaria S, Gopinathan G , et al. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remo-deling: potential applications as a homing factor in dental pulp regeneration[J]. Stem Cell Rev, 2013,9(5):655-667.
doi: 10.1007/s12015-013-9442-7 pmid: 23703692 |
| [16] |
Kim JJ, Bae WJ, Kim JM , et al. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells[J]. J Biomater Appl, 2014,28(7):1069-1078.
doi: 10.1177/0885328213495903 |
| [17] |
Liu H, Ding X, Zhou G , et al. Electrospinning of nanofibers for tissue engineering applications[J]. J Nanomater, 2013,47(2013):63-72.
doi: 10.1155/2013/495708 |
| [18] |
Huang GT, Gronthos S, Shi S . Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine[J]. J Dent Res, 2009,88(9):792-806.
doi: 10.1177/0022034509340867 pmid: 2830488 |
| [19] |
Kumar G, Tison CK, Chatterjee K , et al. The determination of stem cell fate by 3D scaffold structures through the control of cell shape[J]. Biomaterials, 2011,32(35):9188-9196.
doi: 10.1016/j.biomaterials.2011.08.054 pmid: 3428125 |
| [20] |
Chen CS, Mrksich M, Huang S , et al. Geometric control of cell life and death[J]. Science, 1997,276(5317):1425-1428.
doi: 10.1126/science.276.5317.1425 |
| [21] | Folkman J, Moscona A . Role of cell shape in growth control[J]. Nature, 1978,273(5661):345-349. |
| [22] |
McBeath R, Pirone DM, Nelson CM , et al. Cell shape, cytoske-letal tension, and RhoA regulate stem cell lineage commitment[J]. Developmental Cell, 2004,6(4):483-495.
doi: 10.1016/S1534-5807(04)00075-9 |
| [23] |
Lee JH, Lee JW, Khang G , et al. Interaction of cells on chargeable functional group gradient surfaces[J]. Biomaterials, 1997,18(4):351-358.
doi: 10.1016/S0142-9612(96)00128-7 |
| [24] |
Khorasani MT, Mirzadeh H, Irani S . Plasma surface modification of poly (-lactic acid) and poly (lactic-co-glycolic acid) films for improvement of nerve cells adhesion[J]. Radiat Phys Chem, 2008,77(3):280-287.
doi: 10.1016/j.radphyschem.2007.05.013 |
| [1] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [2] | Lei WANG,Xiang-shu JIN,Hui-jun DONG,Guo-min OU,Xin-yuan LAI,Hui ZHUANG,Tong LI,Kuan-hui XIANG. Establishment of a reporter system for estimating activation of human hepatic stellate cells based on COL1A1 promoter and enhanced green fluorescent protein [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 876-885. |
| [3] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [4] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [5] | MA Xin-rong,ZHU Xiao-ming,LI Jing,LI De-li,LI He-ping,TAN Jian-guo. Effect of a novel radio-frequency atmospheric-pressure glow discharge plasma jet treatment on crosslinking of dentin collagen [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 83-88. |
| [6] | Chun-ling CAO,Cong-chong YANG,Xiao-zhong QU,Bing HAN,Xiao-yan WANG. Effects of the injectable glycol-chitosan based hydrogel on the proliferation and differentiation of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 10-17. |
| [7] | Ping WANG,Jing SONG,Xiang-yu FANG,Xin LI,Xu LIU,Yuan JIA,Zhan-guo LI,Fan-lei HU. Role of erythroblast-like Ter cells in the pathogenesis of collagen-induced arthritis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 445-450. |
| [8] | Xiao-ming ZHU,Xuan QI,De-li LI,Yu-wei ZHANG,He-ping LI,Jian-guo TAN. Effect of a novel cold atmospheric plasma jet treatment with different temperatures on resin-dentin bonding [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 43-48. |
| [9] | Rong LI,Ke-long CHEN,Yong WANG,Yun-song LIU,Yong-sheng ZHOU,Yu-chun SUN. Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 115-119. |
| [10] | ZHANG Hui, LI Liang, WANG Qian, GAN Hong-Quan, WANG Hui, BI Cheng, LI Qi-Jia, WANG Zhi-Qiang. Influence of BMP-7 on chondrocyte secretion and expression of Col-Ⅱ,AGG and Sox9 mRNA in porous tantalum-chondrocyte composites in vitro [J]. Journal of Peking University(Health Sciences), 2015, 47(2): 219-225. |
| [11] | 欧Meng-恩 , ZHANG Xiao, LIU Yun-Song, GE Yan-Jun, ZHOU Yong-Sheng. Ectopic osteogenesis of stromal cell-derived factor 1 combined with simvastatin loaded collagen scaffold in vivo [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 47-51. |
| [12] | LIU Yan, FU Yu, LIU Shuai, ZHOU Yan-heng. Effects of microstructure of mineralized collagen scaffolds on cell morphology of MG 63 [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 19-24. |
|
||