Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (2): 234-238. doi: 10.19723/j.issn.1671-167X.2019.02.006
Previous Articles Next Articles
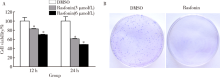
Rasfonin inhibits proliferation and migration of osteosarcoma 143B cells
Fan ZHANG1,2,Tai-qiang YAN1,∆( ),Wei GUO1
),Wei GUO1
- 1. Musculoskeletal Tumor Center, Peking University People’s Hospital, Beijing 100044, China;
2. Department of Bone and Soft Tissue, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China
CLC Number:
- R738.1
| [1] |
Osasan S, Zhang M, Shen F , et al. Osteogenic sarcoma: a 21st century review[J]. Anticancer Res, 2016,36(9):4391-4398.
doi: 10.21873/anticanres |
| [2] |
Shaikh AB, Li F, Li M , et al. Present advances and future perspectives of molecular targeted therapy for osteosarcoma[J]. Int J Mol Sci, 2016,17(4):506.
doi: 10.3390/ijms17040506 |
| [3] |
Tomikawa T, Shin-Ya K, Furihata K , et al. Rasfonin, a new apoptosis inducer in ras-dependent cells from Talaromyces sp.[J]. J Antibiot (Tokyo), 2000,53(8):848-850.
doi: 10.7164/antibiotics.53.848 |
| [4] |
Xiao Z, Li L, Li Y , et al. Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice[J]. Cell Death Dis, 2014,5(5):e1241.
doi: 10.1038/cddis.2014.213 |
| [5] |
Lu Q, Yan S, Sun H , et al. Akt inhibition attenuates rasfonin-induced autophagy and apoptosis through the glycolytic pathway in renal cancer cells[J]. Cell Death Dis, 2015,6(12):e2005.
doi: 10.1038/cddis.2015.344 |
| [6] |
Ouyang L, Shi Z, Zhao S , et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis[J]. Cell Prolif, 2012,45(6):487-498.
doi: 10.1111/cpr.2012.45.issue-6 |
| [7] |
Hale AN, Ledbetter DJ, Gawriluk TR , et al. Autophagy: regulation and role in development[J]. Autophagy, 2013,9(7):951-972.
doi: 10.4161/auto.24273 |
| [8] |
Levine B, Yuan J . Autophagy in cell death: an innocent convict?[J]. J Clin Invest, 2005,115(10):2679-2688.
doi: 10.1172/JCI26390 |
| [9] |
Eisenberg-Lerner A, Bialik S, Simon HU , et al. Life and death partners: apoptosis, autophagy and the cross-talk between them[J]. Cell Death Differ, 2009,16(7):966-975.
doi: 10.1038/cdd.2009.33 |
| [10] | 燕太强, 梁伟民, 郭卫 . 骨肉瘤的诊疗和研究进展[J]. 中华临床医师杂志: 电子版, 2012(17):4988-4990. |
| [11] |
Wang W, Sun H, Che Y , et al. Rasfonin promotes autophagy and apoptosis via upregulation of reactive oxygen species (ros)/jnk pathway[J]. Mycology, 2016,7(2):64.
doi: 10.1080/21501203.2016.1170073 |
| [12] |
Galluzzi L, Vitale I, Abrams JM , et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012[J]. Cell Death Differ, 2012,19(1):107-120.
doi: 10.1038/cdd.2011.96 |
| [13] |
Kanzawa T, Germano IM, Komata T , et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells[J]. Cell Death Differ, 2004,11(4):448-457.
doi: 10.1038/sj.cdd.4401359 |
| [14] |
Shinojima N, Yokoyama T, Kondo Y , et al. Roles of the akt/mtor/p70s6k and erk1/2 signaling pathways in curcumin-induced autophagy[J]. Autophagy, 2007,3(6):635-637.
doi: 10.4161/auto.4916 |
| [15] |
曹珮, 姜学军, 席志军 . 舒尼替尼通过抑制 Akt/mTOR 信号通路诱导肾癌细胞自噬[J]. 北京大学学报(医学版), 2016,48(4):584-589.
doi: 10.3969/j.issn.1671-167X.2016.04.003 |
| [1] | Yao ZHANG,Jinxin GUO,Shijia ZHAN,Enyu HONG,Hui YANG,Anna JIA,Yan CHANG,Yongli GUO,Xuan ZHANG. Role and mechanism of cysteine and glycine-rich protein 2 in the malignant progression of neuroblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 495-504. |
| [2] | Lei WANG,Tian-dong HAN,Wei-xing JIANG,Jun LI,Dao-xin ZHANG,Ye TIAN. Comparison of safety and effectiveness of active migration technique and in situ lithotripsy technique in the treatment of 1-2 cm upper ureteral calculi by flexible ure-teroscopy [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 553-557. |
| [3] | Li-ye LAI,Chang-song DOU,Cui-na ZHI,Jie CHEN,Xue MA,Peng ZHAO,Bi-yun YAO. Curcumin alleviates the manganese-induced neurotoxicity by promoting autophagy in rat models of manganism [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 400-411. |
| [4] | YANG Duo,ZHOU Xin-na,WANG Shuo,WANG Xiao-li,YUAN Yan-hua,YANG Hua-bin,GENG Hui-zhen,PENG Bing,LI Zi-bo,LI Bin,REN Jun. Assessment of lymphocytic function in vitro stimulated by specific tumor polypeptide combined with dendritic cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1094-1098. |
| [5] | Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 893-899. |
| [6] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [7] | SUN Jing, SONG Wei-dong, YAN Si-yuan, XI Zhi-jun. Chloroquine inhibits viability of renal carcinoma cells and enhances sunitinib-induced caspase-dependent apoptosis [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 778-784. |
| [8] | WANG Zi-cheng, CHENG Li, LV Tong-de, SU Li, LIN Jian, ZHOU Li-qun. Inflammatory priming adipose derived stem cells significantly inhibit the proliferation of peripheral blood mononuclear cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 590-594. |
| [9] | TANG Xu, ZHAO Wei-hong, SONG Qin-qin, YIN Hua-qi, DU Yi-qing, SHENG Zheng-zuo, WANG Qiang, ZHANG Xiao-wei, LI Qing, LIU Shi-jun, XU Tao. Influence of SOX10 on the proliferation and invasion of prostate cancer cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 602-606. |
| [10] | WANG Xiao-tong, RAO Nan-quan, FANG Teng-jiao-zi, ZHAO Yu-ming, GE Li-hong. Comparison of the properties of CD146 positive and CD146 negative subpopulations of stem cells from human exfoliated deciduous teeth [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 284-292. |
| [11] | CHEN Wei, HU Fan-lei, LIU Hong-jiang, XU Li-ling, LI Ying-ni, LI Zhan-guo. Myeloid-derived suppressor cells promoted autologous B cell proliferation in rheumatoid arthritis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 819-823. |
| [12] | CAI Yi, GUO Hao, LI Han-zhong, WANG Wen-da, ZHANG Yu-shi. MicroRNA differential expression profile in tuberous sclerosis complex cell line TSC2-/- MEFs and normal cell line TSC2+/+ MEFs [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 580-584. |
| [13] | SIMA Zi-han, HONG Ying-ying, LI Tie-jun△. Effects of PTCH1 mutations on the epithelial proliferation derived from keratocystic odontogenic tumour [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 522-526. |
| [14] | GAO Xiang, CHEN Xiang-mei, ZHANG Ting, ZHANG Jing, CHEN Mo, GUO Zheng--yang, SHI Yan-yan, LU Feng-min, DING Shi-gang. Relationship between macrophage capping protein and gastric cancer cell’s proliferation and migration ability [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 489-494. |
| [15] | CAI Yuan-fa, ZHANG Hua-ming, NIU Wen-yi, ZOU Yong-qiu, MA De-fu. Effects of equol on colon cancer cell proliferation [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 383-387. |
|
||