Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (2): 228-233. doi: 10.19723/j.issn.1671-167X.2019.02.005
Previous Articles Next Articles
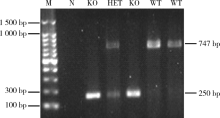
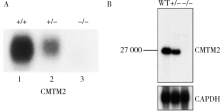
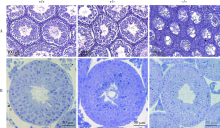
CMTM2 is involved in spermiogenesis in mice
Xiao-wei ZHANG1,Hua-qi YIN1,Qing LI1,Yong-ping ZHAO2,BRANDES Kite3,Wen-jun BAI1,Tao XU1,∆( )
)
- 1. Department of Urology, Peking University People’s Hospital, Beijing 100044, China;
2. Reproductive Medicine Center, Peking University People’s Hospital, Beijing 100044, China;
CLC Number:
- R698
| [1] |
Zhang Y, Xiao F, Lu S , et al. Research trends and perspectives of male infertility: a bibliometric analysis of 20 years of scientific literature[J]. Andrology, 2016,4(6):990-1001.
doi: 10.1111/andr.12204 |
| [2] |
Kumar N, Singh AK . Trends of male factor infertility, an important cause of infertility: a review of literature[J]. J Hum Reprod Sci, 2015,8(4):191-196.
doi: 10.4103/0974-1208.170370 |
| [3] | Cho C, Willis WD, Goulding EH , et al. Haploinsufficiency of protamine-1 or-2 causes infertility in mice[J]. Nat Genet, 2001,28(1):82-86. |
| [4] |
Pan J, Goodheart M, Chuma S , et al. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis[J]. Development, 2005,132(18):4029-4039.
doi: 10.1242/dev.02003 |
| [5] |
Yan W . Male infertility caused by spermiogenic defects: lessons from gene knockouts[J]. Mol Cell Endocrinol, 2009,306(1/2):24-32.
doi: 10.1016/j.mce.2009.03.003 |
| [6] |
Liu G, Xin ZC, Chen L , et al. Expression and localization of CKLFSF2 in human spermatogenesis[J]. Asian J Androl, 2007,9(2):189-198.
doi: 10.1111/ajan.2007.9.issue-2 |
| [7] |
Han W, Ding P, Xu M , et al. Identification of eight genes encoding chemokine-like factor superfamily members 1-8 (CKLFSF1-8) by in silico cloning and experimental validation[J]. Genomics, 2003,81(6):609-617.
doi: 10.1016/S0888-7543(03)00095-8 |
| [8] |
Han W, Lou Y, Tang J , et al. Molecular cloning and characterization of chemokine-like factor 1 (CKLF1), a novel human cytokine with unique structure and potential chemotactic activity[J]. Biochem J, 2001,357(Ptl):127-135.
doi: 10.1042/bj3570127 |
| [9] |
Shi S, Rui M, Han W , et al. CKLFSF2 is highly expressed in testis and can be secreted into the seminiferous tubules[J]. Int J Biochem Cell Biol, 2005,37(8):1633-1640.
doi: 10.1016/j.biocel.2004.04.028 |
| [10] |
Zhang XD, Yin LL, Zheng Y , et al. Expression of a novel beta adaptin subunit mRNA splice variant in human testes[J]. Asian J Androl, 2005,7(2):179-188.
doi: 10.1111/ajan.2005.7.issue-2 |
| [11] |
Noormets K, Kõks S, Kavak A , et al. Male mice with deleted Wolframin (Wfs1) gene have reduced fertility [J]. Reprod Biol Endocrinol, 2009,7:82.
doi: 10.1186/1477-7827-7-82 |
| [12] | Ramalho-Santos J, Moreno RD . Targeting and fusion proteins during mammalian spermiogenesis[J]. Biol Res, 2001,34(2):147-152. |
| [13] |
Sánchez-Pulido L, Martín-Belmonte F, Valencia A , et al. MARVEL: a conserved domain involved in membrane apposition events[J]. Trends Biochem Sci, 2002,27(12):599-601.
doi: 10.1016/S0968-0004(02)02229-6 |
| [14] |
Centola GM . Semen assessment[J]. Urol Clin North Am, 2014,41(1):163-167.
doi: 10.1016/j.ucl.2013.08.007 |
| [15] |
Parmegiani L, Cognigni GE, Filicori M . Sperm selection: effect on sperm DNA quality[J]. Adv Exp Med Biol, 2014,791:151-172.
doi: 10.1007/978-1-4614-7783-9 |
| [16] |
Skakkebaek NE, Jørgensen N, Main KM , et al. Is human fecundity declining[J]. Int J Androl, 2006,29(1):2-11.
doi: 10.1111/ija.2006.29.issue-1 |
| [17] |
Shamsi MB, Kumar K, Dada R . Genetic and epigenetic factors: Role in male infertility[J]. Indian J Urol, 2011,27(1):110-120.
doi: 10.4103/0970-1591.78436 |
| [18] | 刘振华, 谢京, 萧云备 , 等. CMTM2改善环磷酰胺致转基因小鼠模型生殖毒性作用并影响StAR蛋白的表达[J]. 中华男科学杂志, 2013,19(3):210-213. |
| [19] | 刘振华, 萧云备, 张晓威 , 等. CMTM2转基因小鼠的构建及其血清睾酮水平的变化[J]. 中华男科学杂志, 2012,18(6):483-486. |
| [20] |
Walker WH, Habener JE . Role of transcription factors CREB and CREM in cAMP-regulated transcription during spermatogenesis[J]. Trends Endocrinol Metab, 1996,7(4):133-138.
doi: 10.1016/1043-2760(96)00035-5 |
| [21] |
Sassone-Corsi P . CREM: a master-switch governing male germ cells differentiation and apoptosis[J]. Semin Cell Dev Biol, 1998,9(4):475-482.
doi: 10.1006/scdb.1998.0200 |
| [22] | Schmidt EE, Schibler U . High accumulation of components of the RNA polymerase II transcription machinery in rodent spermatids[J]. Development, 1995,121(8):2373-2383. |
| [23] |
Kotaja N . MicroRNAs and spermatogenesis[J]. Fertil Steril, 2014,101(6):1552-1562.
doi: 10.1016/j.fertnstert.2014.04.025 |
| [1] | Hailong HE,Qing LI,Tao XU,Xiaowei ZHANG. Construction of a predictive model for postoperative pain relief after microscopic spermatic cord surgery for spermatic cord pain [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 646-655. |
| [2] | Wen-hao TANG,Chen-yao DENG,Jiang-man GAO,Zhi-chao LUO,Han WU,Sen-lin TIAN,Nan WEI,Bin Li,Qian-cheng ZHAO,Jian-fei SONG,Liang ZHANG,Lu-lin MA,Hui JIANG. Seasonal variations influenced the semen quality of the sperm donor in Beijing area [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 658-662. |
| [3] | Jia-ming MAO,Lian-ming ZHAO,De-feng LIU,Hao-cheng LIN,Yu-zhuo YANG,Hai-tao ZHANG,Kai HONG,Rong LI,Hui JIANG. Analysis of clinical outcome of synchronous micro-dissection testicular sperm extraction and intracytoplasmic sperm injection in male infertility with Y chromosome azoospermia factor c region deletion [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 652-657. |
| [4] | PENG Jing,FANG Dong,ZHANG Zhi-chao,GAO Bing,YUAN Yi-ming,TANG Yuan,SONG Wei-dong,CUI Wan-shou. Testosterone levels in patients with varicocele and azoospermia [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 294-298. |
| [5] | FENG Ke,NI Jing-jing,XIA Yan-qing,QU Xiao-wei,ZHANG Hui-juan,WAN Feng,HONG Kai,ZHANG Cui-lian,GUO Hai-bin. Genetic analysis of three cases of acephalic spermatozoa syndrome caused by SUN5 mutation and the outcome of assisted reproductive technology [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 803-807. |
| [6] | Hong-bin WANG,Lian-ming ZHAO,Kai HONG,Jia-ming MAO,De-feng LIU,Hao-cheng LIN,Hui JIANG. Transurethral seminal vesiculoscopy in treatment of oligoasthenozoospermia secondary incomplete ejaculatory duct obstruction: A report of 8 cases [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 642-645. |
| [7] | Lian-ming ZHAO,Hui JIANG,Kai HONG,Hao-cheng LIN,Wen-hao TANG,De-feng LIU,Jia-ming MAO,Zhe ZHANG,Sheng-li LIN,Lu-lin MA. Analysis of intratesticular condition in micro-dissection testicular sperm extraction era [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 632-635. |
| [8] | DAI Xiao-wei, XU Ying, ZHENG Lian-wen, LI Ling-yun, LI Dan-dan1 TAN Xin, GAO Fei, WANG Yan, WU Gui-jie. Analysis of chromosome in 1 324 patients with oligozoospermia or azoosperm [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 774-777. |
| [9] | MAO Jia-ming, LIU De-feng,ZHAO Lian-ming,HONG Kai, ZHANG Li, MA Lu-lin, JIANG Hui, QIAO Jie. Effect of testicular puncture biopsy on the success rate of microdissection testicular sperm extraction for idiopathic non-obstructive azoospermia [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 613-616. |
| [10] | ZHANG Xiao-wei, LAN Ke, YANG Wen-bo, LI Qing, ZHAO Yong-ping, YIN Hua-qi, Kite Brandes, BAI Wen-jun, XU Tao. Expression and localization of transmembrane protein CMTM2 in human testis and sperm [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 575-579. |
| [11] | ZHANG Xiao-wei, DUN Yao-jun, TANG Xu, YIN Hua-qi, HU Zhi-ping, ZHAO Yong-ping, XU Tao, LI Qing. Expression of chemokine like factor-like myelin and lymphocyte and related proteins for vesicle trafficking and membrane link transmembrane domaincontaining protein 2 in rats with varicocele [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 579-583. |
| [12] | YIN Zhuo, YANG Jin-Rui, WANG Zhao, WEI Yong-Bao, YAN Bin, ZHOU Ke-Qin. Application of scrotoscope in the diagnosis and treatment of testicular and epididymal diseases [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 648-652. |
| [13] | WEN Meng-Meng, ZHU Guang-Rong, WANG Hai-Xue. Association between obesity and age at spermarche among Chinese Han boys aged 11-18 years [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 406-409. |
|
||