Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (2): 239-244. doi: 10.19723/j.issn.1671-167X.2019.02.007
Previous Articles Next Articles
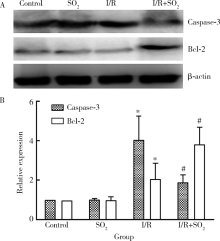
Effects of sulfur dioxide on alveolar macrophage apoptosis in acute lung injury induced by limb ischemia/reperfusion in rats
Yan-rui ZHAO,Yang LIU,Dong WANG,Wen-rui LV,Jun-lin ZHOU( )
)
- Department of Orthopedics, Beijing Chao-yang Hospital, Capital Medical University, Beijing 100020, China
CLC Number:
- R364.12
| [1] |
Steiger AK, Yang Y, Royzen M , et al. Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H2S)[J]. Chem Commun (Camb), 2017,53(8):1378-1380.
doi: 10.1039/C6CC09547J |
| [2] |
Steiger AK, Pardue S, Kevil CG , et al. Self-immolative thiocarbamates provide access to triggered H2S donors and analyte replacement fluorescent probes[J]. J Am Chem Soc, 2016,138(23):7256-7259.
doi: 10.1021/jacs.6b03780 |
| [3] |
Aggarwal NR, D’Alessio FR, Tsushima K , et al. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice[J]. Am J Physiol Lung Cell Mol Physiol, 2010,298(3):L371-L381.
doi: 10.1152/ajplung.00308.2009 |
| [4] |
Howard KM . Differential expression of platelet-activating factor acetylhydrolase in lung macrophages[J]. Am J Physiol Lung Cell Mol Physiol, 2009,297(6):L1141-L1150.
doi: 10.1152/ajplung.00022.2009 |
| [5] | Z’Graggen BR, Tornic J, Muller-Edenborn B , et al. Acute lung injury: apoptosis in effector and target cells of the upper and lower airway compartment[J]. Clin Exp Immunol, 2010,161(2):324-331. |
| [6] |
Meng Z, Liu Y . Cell morphological ultrastructural changes in va-rious organs from mice exposed by inhalation to sulfur dioxide[J]. Inhal Toxicol, 2007,19(6-7):543-551.
doi: 10.1080/08958370701271373 |
| [7] | Ubuka T, Yuasa S, Ohta J , et al. Formation of sulfate from L-cysteine in rat liver mitochondria[J]. Acta Med Okayama, 1990,44(2):55-64. |
| [8] | 赵彦瑞, 刘洋, 王东 , 等. PI3KAkt和JAK2STAT3信号转导通路在SO2抗大鼠肢体缺血再灌注致急性肺损伤中的作用[J]. 中华病理生理杂志, 2015,31(11):2076-2082. |
| [9] |
Huang XL, Liu Y, Zhou JL , et a1. Role of sulfur dioxide in acute lung injury following limb ischemia/reperfusion in rats[J]. J Biochem Mol Toxicol, 2013,27(8):389-397.
doi: 10.1002/jbt.2013.27.issue-8 |
| [10] |
Zhao YR, Wang D, Liu Y , et al. The PI3K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways mediate the protection of SO2 against acute lung injury induced by limb ischemia/reperfusion in rats[J]. J Physiol Sci, 2016,66(3):229-239.
doi: 10.1007/s12576-015-0418-z |
| [11] |
Cohen SM, Siddiqi FA, Darakchiev B , et al. Attenuation of acute lung injury caused by hind-limb ischemia-reperfusion injury by butyrolactone anti-inflammatory agent FLl003[J]. J Trauma, 1997,43(2):247-252.
doi: 10.1097/00005373-199708000-00007 |
| [12] | Javadov S, Choi A, Rajapurohitam V , et al. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associa-ted with attenuation of the mitochondrial permeability transition[J]. Cardiovasc Res, 2008,77(2):416-424. |
| [13] | Kaltenbach JP, Jennings RB . Metabolism of ischemic cardiac muscle[J]. Circ Res, 1960(8):207-213. |
| [14] |
Ward PA . Editorial commentary: New strategies for treatment of humans with acute lung injury/acute respiratory distress syndrome[J]. Clin Infect Dis, 2015,60(4):596-597.
doi: 10.1093/cid/ciu892 |
| [15] |
Tauber AI . Metchnikoff and the phagocytosis theory[J]. Nat Rev Mol Cell Biol, 2003,4(11):897-901.
doi: 10.1038/nrm1244 |
| [16] |
Ovchinnikov, Dmitry A . Macrophages in the embryo and beyond: Much more than just giant phagocytes[J]. Genesis, 2008,46(9):447-462.
doi: 10.1002/dvg.v46:9 |
| [17] | Yong Y, Matthew S, Wittwer J , et al. Dichamanetin inhibits cancer cell growth by affecting ROS-related signaling components through mitochondrial-mediated apoptosis[J]. Anticancer Res, 2013,33(12):5349-5355. |
| [1] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [2] | Zhi-wei LIU,Peng LIU,Fan-xing MENG,Tian-shui LI,Ying WANG,Jia-qi GAO,Zuo-yi ZHOU,Cong WANG,Bin ZHAO. Regulative effects of endogenous sulfur dioxide on oxidant stress in myocardium of rat with sepsis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 582-586. |
| [3] | WANG Si-wen,YOU Peng-yue,LIU Yu-hua,WANG Xin-zhi,TANG Lin,WANG Mei. Efficacy of two barrier membranes and deproteinized bovine bone mineral on bone regeneration in extraction sockets: A microcomputed tomographic study in dogs [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 364-370. |
| [4] | Ying-yi CHEN,Zi-qi HU,Tian-qian HUI,He LIU. Enhancer of zeste homolog 2 affects dental pulp inflammation by regulating macrophage chemotaxis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 18-23. |
| [5] | Ming-xin MAO,Li XU,Wu-di JING,Xiao XU,Jian-xia HOU,Xiao-tong LI,Xiao-xia WANG. Alveolar crest and relevant analysis of labial side of anterior teeth on skeletal Angle class Ⅲ patients [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 77-82. |
| [6] | Guo-zhong LIN,Zhen-yu WANG,Bin LIU,Shao-min YANG. Intraspinal metastasis of alveolar rhabdomyosarcoma: A case report [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1165-1168. |
| [7] | Li-ping ZHAO,Wen-jie HU,Tao XU,Ya-lin ZHAN,Yi-ping WEI,Min ZHEN,Cui WANG. Two procedures for ridge preservation of molar extraction sites affected by severe bone defect due to advanced periodontitis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 579-585. |
| [8] | WU Xiao-jing, LI Min, ZHAN Qing-yuan. Exogenous lipid pneumonia with hyperpyrexia: a case report [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 921-923. |
| [9] | ZHANG You-wen, XIN Tian-yi, JIAO Jian,ZHOU Yan-heng, SHI Jie. Extraction-orthodontic treatment on patients with chronicperiodontitis: a preliminary study [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 308-313. |
| [10] | MA Jing, JIANG Jiu-hui. Morphological analysis of alveolar bone of anterior mandible in high-angle skeletal class Ⅱ and class Ⅲ malocclusions assessed with cone-beam computed tomography [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 98-103. |
| [11] | JIA Peng-chen, YANG Gang, HU Wen-jie, ZHAO Yi-jiao, LIU Mu-qing. Preliminary study on the accuracy of infrabony root surface area of single-root teeth by periapical films [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 91-97. |
| [12] | XU Xiao, XU Li, JIANG Jiu-hui, WU Jia-qi, LI Xiao-tong, JING Wu-di. Accuracy analysis of alveolar dehiscence and fenestration of maxillary anterior teeth of Angle class Ⅲ by cone-beam CT [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 104-109. |
| [13] | CAO Jie, MENG Huan-xin. Evaluation of using cone beam computed tomography as a regular test before and after periodontal regenerative surgery#br# [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 110-116. |
| [14] | ZHAN Ya-lin, HU Wen-jie, XU Tao, ZHEN Min, LU Rui-fang. Histomorphometric evaluation of ridge preservation after molar tooth extraction [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 169-175. |
| [15] | SHEN Xiao, SHI Jie, XU Li, JIAO Jian, LU Rui-fang, MENG Huan-xin. Clinical evaluation of periodontal-orthodontic treatment in patients with aggressive periodontitis and malocclusion [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 60-066. |
|
||