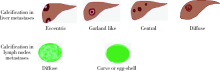
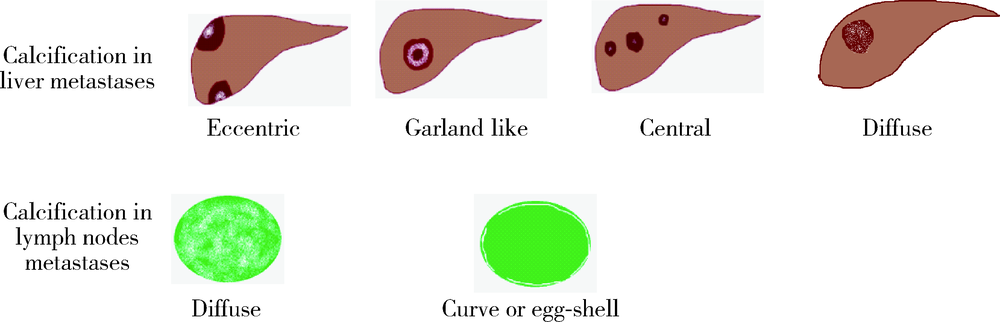
| [1] |
Chen W, Zheng R, Baade PD , et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016,66(2):115-132.
|
| [2] |
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2018[J]. CA Cancer J Clin, 2018,68(1):7-30.
|
| [3] |
Eisenhauer EA, Therasse P, Bogaerts J , et al. New response eva-luation criteria in solid tumours: Revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009,45(2):228-247.
|
| [4] |
Easson AM, Barron PT, Cripps C , et al. Calcification in colorectal hepatic metastases correlates with longer survival[J]. J Surg Oncol, 1996,63(4):221-225.
|
| [5] |
Hale HL, Husband JE, Gossios K , et al. CT of calcified liver metastases in colorectal carcinoma[J]. Clin Radiol, 1998,53(10):735-741.
|
| [6] |
姜昊, 姜慧杰, 潘文彬 , 等. 不同来源肝转移瘤多层螺旋CT影像学特征的分析[J]. 中华结直肠疾病电子杂志, 2017,6(1):41-45.
|
| [7] |
Roy B, Verma S, Awasthi R , et al. Correlation of phase values with CT Hounsfield and R2*values in calcified neurocysticercosis[J]. J Magn Reson Imaging, 2011,34(5):1060-1064.
|
| [8] |
Giachelli CM . Ectopic calcification:Gathering hard facts about soft tissue mineralization[J]. Am J Pathol, 1999,154(3):671-675.
|
| [9] |
Agarwal A, Yeh BM, Breiman RS , et al. Peritoneal calcification: Causes and distinguishing features on CT[J]. AJR Am J Roentgenol, 2004,182(2):441-445.
|
| [10] |
邓祥春, 郑波, 童朝阳 , 等. 多层螺旋CT对黏液性与非黏液性结直肠癌的鉴别诊断价值[J]. 中国CT和MRI杂志, 2015,13(8):80-83.
|
| [11] |
Sweeney DJ, Low VH, Robbins PD , et al. Calcified lymph node metastases in adenocarcinoma of the colon[J]. Australas Radiol, 1994,38(3):233-234.
|
| [12] |
Caskey CI, Fishman EK . Computed tomography of calcified meta-stases to skeletal muscle from adenocarcinoma of the colon[J]. J Comput Tomogr, 1988,12(3):199-202.
|
| [13] |
Yoshikawa H, Kameyama M, Ueda T , et al. Ossifying intramuscular metastasis from colon cancer: Report of a case[J]. Dis Colon Rectum, 1999,42(9):1225-1227.
|
| [14] |
Yu MH, Kim YJ, Park HS , et al. Imaging patterns of intratumoral calcification in the abdominopelvic cavity[J]. Korean J Radiol, 2017,18(2):323-335.
|
| [15] |
Günhan-Bilgen I, Oktay A . Management of microcalcifications developing at the lumpectomy bed after conservative surgery and radiation therapy[J]. AJR Am J Roentgenol, 2007,188(2):393-398.
|
| [16] |
Goyer P, BenoistE S, Julie C , et al. Complete calcification of colorectal liver metastases on imaging after chemotherapy does not indicate sterilization of disease[J]. J Visc Surg, 2012,149(4):E271-E274.
|
| [17] |
Cheng JM, Tirumani SH, Kim KW , et al. Malignant abdominal rocks: where do they come from?[J]. Cancer Imaging, 2013,13(4):527-539.
|
 )
)