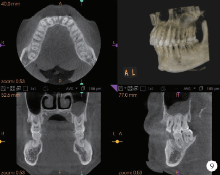
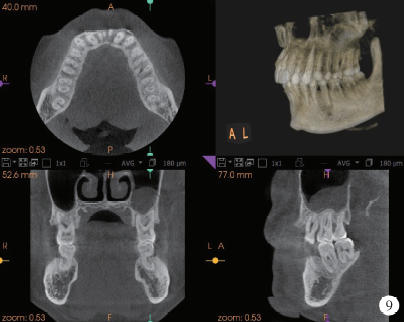
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (2): 346-352. doi: 10.19723/j.issn.1671-167X.2020.02.024
Previous Articles Next Articles
Effect of concentrated growth factors combined with guided tissue regeneration in treatment of classⅡ furcation involvements of mandibular molars
Fei LI,Jing QIAO,Jin-yu DUAN,Yong ZHANG,Xiu-jing WANG( )
)
- First Clinical Division, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100034, China
CLC Number:
- R781.3
| [1] | Janssen KM , Vissink A, de Smit MJ, et al.Lessons to be learned from periodontitis[J]. Curr Opin Rheumatol, 2013,25(2):241-247. |
| [2] | Trombelli L, Farina R . Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration[J]. J Clin Periodontol, 2008,35(8 Suppl):117-135. |
| [3] | Stoecklin-Wasmer C ,Rutjes AW,da Costa BR,et al.Absorbable collagen membranes for periodontal regeneration: a systematic review[J]. J Dent Res, 2013,92(9):773-781. |
| [4] | Susin C, Fiorini T, Lee J , et al. Wound healing following surgical and regenerative periodontal therapy[J]. Periodontol 2000, 2015,68(1):83-98. |
| [5] | Martin P . Wound healing--aiming for perfect skin regeneration[J]. Science, 1997,276(5309):75-81. |
| [6] | Giannobile WV, Finkelman RD, Lynch SE . Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I[J]. J Periodontol, 1994,65(12):1158-1168. |
| [7] | Qiao J, An N, Ouyang X . Quantification of growth factors in different platelet concentrates[J]. Platelets, 2017,28(8):774-778. |
| [8] | Rodella LF, Favero G, Boninsegna R , et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction[J]. Microsc Res Tech, 2011,74(8):772-777. |
| [9] | 乔静, 段晋瑜, 褚祎 , 等. 浓缩生长因子在下颌磨牙Ⅱ度根分叉病变再生治疗中的应用[J]. 北京大学学报(医学版), 2017,49(1):36-42. |
| [10] | Hamp SE, Nyman S, Lindhe J . Periodontal treatment of multiroo-ted teeth. Results after 5 years[J]. J Clin Periodontol, 1975,2(3):126-135. |
| [11] | Qiao J, Duan J, Zhang Y , et al. The effect of concentrated growth factors in the treatment of periodontal intrabony defects [J].Future Sci OA, 2016, 2(4): FS136. |
| [12] | Pirpir C, Yilmaz O, Candirli C , et al. Evaluation of effectiveness of concentrated growth factor on osseointegration[J]. Int J Implant Dent, 2017,3(1):7. |
| [13] | Shyu SS, Fu E, Shen EC . Clinical and microcomputed topography evaluation of the concentrated growth factors as a sole material in a cystic bony defect in alveolar bone followed by dental implantation: a case report[J]. Implant Dent, 2016,25(5):707-714. |
| [14] | Masuki H, Okudera T, Watanebe T , et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF)[J]. Int J Implant Dent, 2016,2(1):19. |
| [15] | Sahin IO, Gokmenoglu C, Kara C . Effect of concentrated growth factor on osteoblast cell response[J]. J Stomatol Oral Maxillofac Surg, 2018,119(6):477-481. |
| [16] | Qin J, Wang L, Sun Y , et al. Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo[J]. Int J Mol Med, 2016,37(2):493-500. |
| [17] | Pontoriero R, Lindhe J, Nyman S , et al. Guided tissue regeneration in degree Ⅱ furcation-involved mandibular molars. A clinical study[J]. J Clin Periodontol, 1988,15(4):247-254. |
| [18] | Needleman I, Tucker R, Giedrys-Leeper E , et al. A systematic review of guided tissue regeneration for periodontai infrabony defects[J]. J Periodont Res, 2002,37(5):380-388. |
| [19] | Behring J, Junker R, Walboomers XF , et al. Toward guided tissue and bone regeneration: morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review[J]. Odontology, 2008,96(1):1-11. |
| [20] | Felipe M, Andrade PF, Grisi MFM , et al. Comparison of two surgical procedures for use of the acellular dermal matrix graft in the treatment of gingival recession: a randomized controlled clinical study[J]. J Periodontol, 2007,78(7):1209-1217. |
| [21] | Pajnigara NG, Kolte AP, Kolte RA , et al. Volumetric assessment of regenerative efficacy of demineralized freeze-dried bone allograft with or without amnion membrane in grade Ⅱ furcation defects: a cone beam computed tomography study[J]. Int J Periodontics Restorative Dent, 2017,37(2):255-262. |
| [22] | Irokawa D, Takeuchi T, Noda K , et al. Clinical outcome of periodontal regenerative therapy using collagen membrane and deproteinized bovine bone mineral: a 2.5-year follow-up study[J]. BMC Res Notes, 2017,10(1):102. |
| [23] | Kini V, Nayak DG, Uppoor AS . A clinical evaluation of biphasic calcium phosphate alloplast with and without a flowable bioabsorbable guided tissue regeneration barrier in the treatment of mandi-bular molar class Ⅱ furcation defects[J]. J Contemp Dent Pract, 2016,17(2):143-148. |
| [24] | Bozkurt A, Apel C, Sellhaus B , et al. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: an in vitro and in vivo study[J]. Clin Oral Implants Res, 2014,25(12):1403-1411. |
| [1] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [2] | Bo-wen LI,Wei-yi WU,Lin TANG,Yi ZHANG,Yu-hua LIU. Barrier effect of improved porcine small intestinal submucosa absorbable membrane on early healing of mandibular defects in rabbits [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 887-892. |
| [3] | ZHAN Ya-lin, HU Wen-jie, XU Tao, ZHEN Min, LU Rui-fang. Histomorphometric evaluation of ridge preservation after molar tooth extraction [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 169-175. |
| [4] | QIAO Jing, DUAN Jin-yu, CHU Yi, SUN Chang-zhou. Effect of concentrated growth factors on the treatment of degree Ⅱ furcation involvements of mandibular molars [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 36-042. |
| [5] | CHEN Fei, PAN Shao-xia, FENG Hai-lan. Distribution and content of transforming growth factor-β1 and vascular endothelial growth factor in each layer of concentrated growth factors [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 860-865. |
|
||