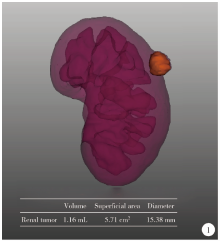
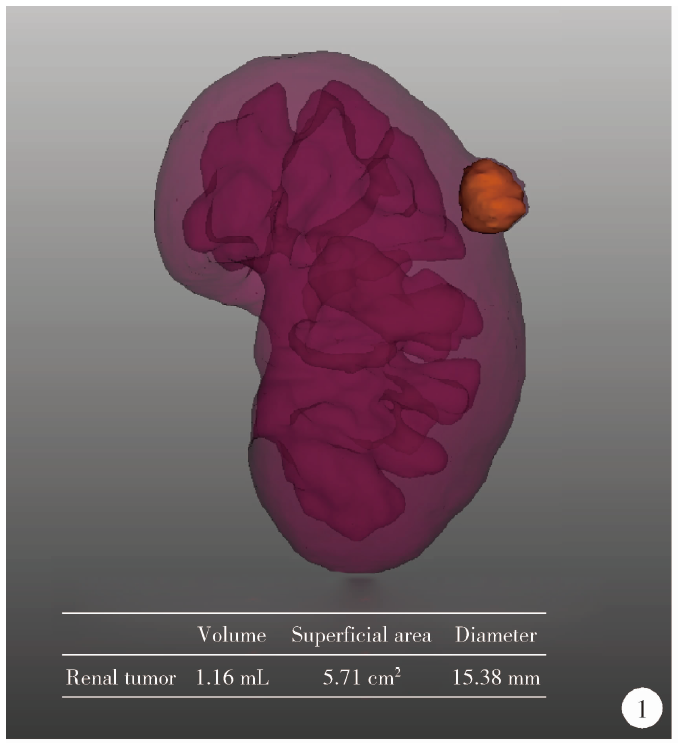
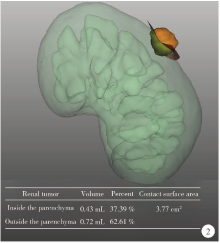
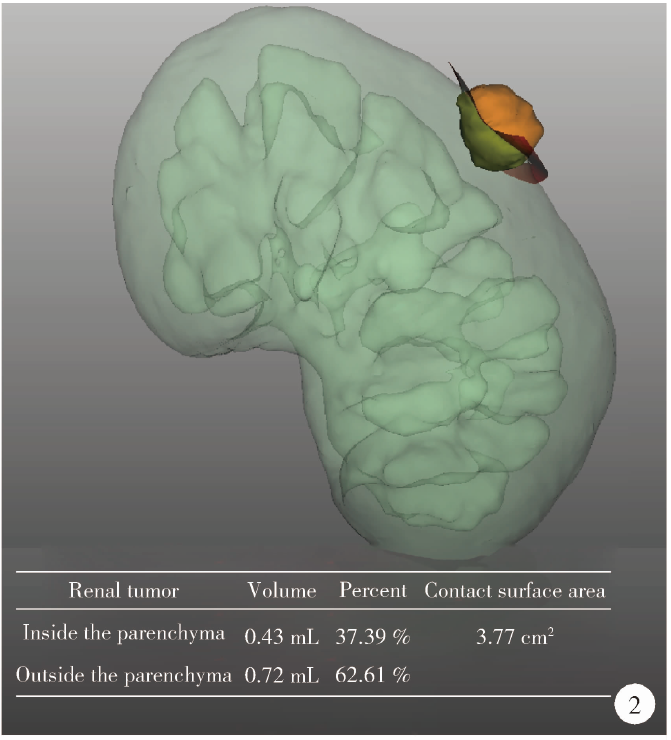
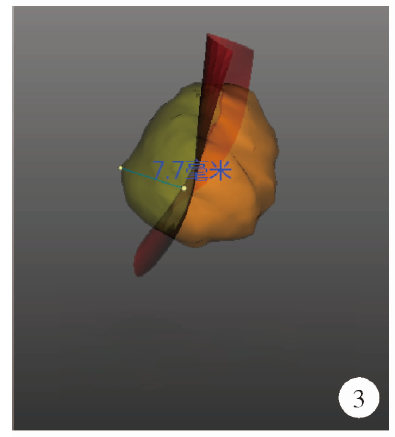
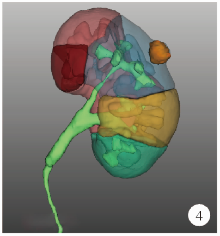
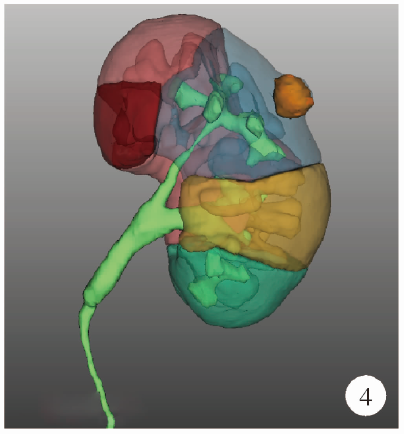
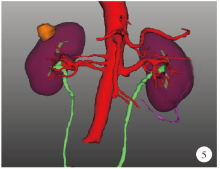
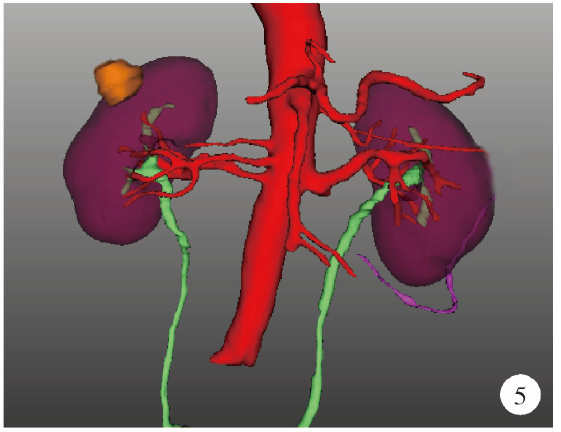
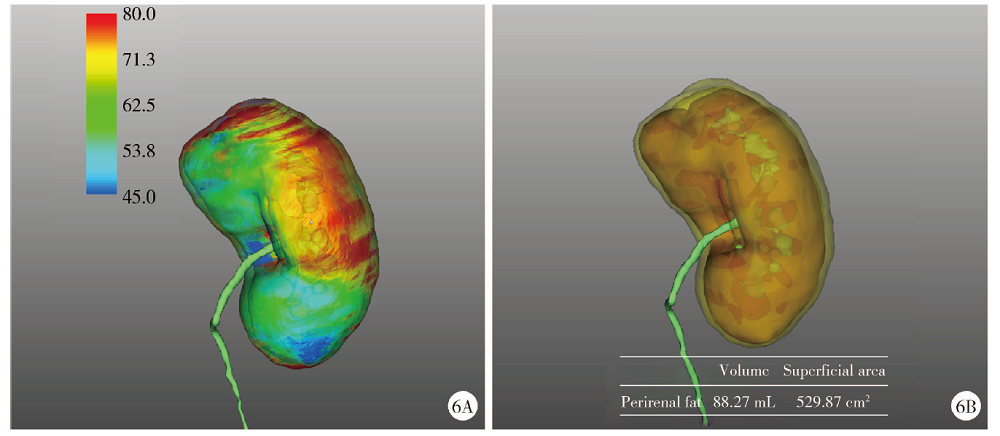
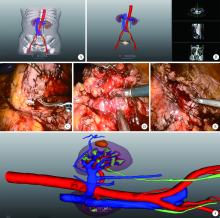
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (3): 613-622. doi: 10.19723/j.issn.1671-167X.2021.03.030
Previous Articles Next Articles
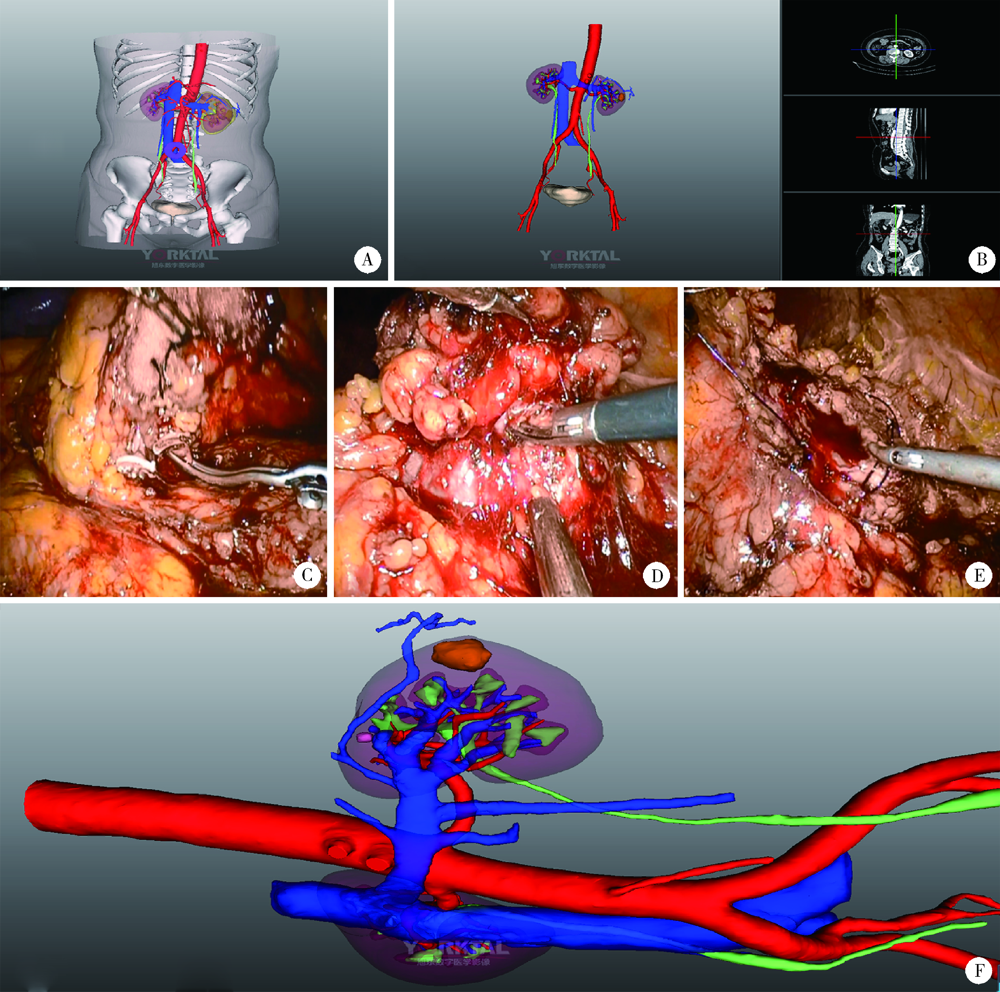
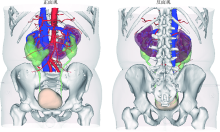
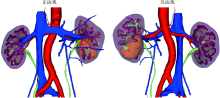
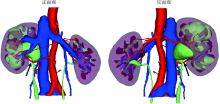
Three dimensional nephrometry system for partial nephrectomy: Our initial exploration
LI Xin-fei1,*,PENG Yi-ji1,*,YU Xiao-teng1,*,XIONG Sheng-wei1,CHENG Si-da1,DING Guang-pu1,YANG Kun-lin1,TANG Qi1,Δ( ),MI Yue1,WU Jing-yun1,ZHANG Peng2,XIE Jia-xin1,HAO Han1,WANG He3,QIU Jian-xing3,YANG Jian4,LI Xue-song1,Δ(
),MI Yue1,WU Jing-yun1,ZHANG Peng2,XIE Jia-xin1,HAO Han1,WANG He3,QIU Jian-xing3,YANG Jian4,LI Xue-song1,Δ( ),ZHOU Li-qun1
),ZHOU Li-qun1
- 1. Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
2. Department of Urology, Emergency General Hospital, Beijing 100028, China
3. Department of Radiology, Peking University First Hospital, Beijing 100034, China
4. Beijing Engineering Research Center for Mixed Reality and Advanced Display Technology, School of Optics and Photonics, Beijing Institute of Technology, Beijing 100081, China
CLC Number:
- R737.11
| [1] |
Rossi SH, Klatte T, Usher-Smith J, et al. Epidemiology and screening for renal cancer[J]. World J Urol, 2018,36(9):1341-1353.
doi: 10.1007/s00345-018-2286-7 |
| [2] |
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2. NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2017,15(6):804-834.
doi: 10.6004/jnccn.2017.0100 |
| [3] |
Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline[J]. J Urol, 2017,198(3):520-529.
doi: 10.1016/j.juro.2017.04.100 |
| [4] |
Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery[J]. Eur Urol, 2009,56(5):786-793.
doi: 10.1016/j.eururo.2009.07.040 |
| [5] |
Kutikov A, Uzzo RG. The R. E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth[J]. J Urol, 2009,182(3):844-853.
doi: 10.1016/j.juro.2009.05.035 |
| [6] |
Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method[J]. J Urol, 2010,183(5):1708-1713.
doi: 10.1016/j.juro.2010.01.005 |
| [7] |
Hew MN, Baseskioglu B, Barwari K, et al. Critical appraisal of the PADUA classification and assessment of the R.E.N.A.L. nephrometry score in patients undergoing partial nephrectomy[J]. J Urol, 2011,186(1):42-46.
doi: 10.1016/j.juro.2011.03.020 |
| [8] | Wadle J, Hetjens S, Winter J, et al. Nephrometry scores: The effect of imaging on routine read-out and prediction of outcome of nephron-sparing surgery[J]. Anticancer Res, 2018,38(5):3037-3041. |
| [9] | Porpiglia F, Amparore D, Checcucci E, et al. Current use of three-dimensional model technology in urology: A road map for personalised surgical planning[J]. Eur Urol Focus, 2018,4(5):652-656. |
| [10] |
Cartiaux O, Paul L, Francq BG, et al. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery[J]. Ann Biomed Eng, 2014,42(1):205-213.
doi: 10.1007/s10439-013-0890-7 pmid: 23963884 |
| [11] |
Wu J, Li Y, Zhang Y. Use of intraoral scanning and 3-dimensional printing in the fabrication of a removable partial denture for a patient with limited mouth opening[J]. J Am Dent Assoc, 2017,148(5):338-341.
doi: 10.1016/j.adaj.2017.01.022 |
| [12] |
Porpiglia F, Amparore D, Checcucci E, et al. Three-dimensional virtual imaging of renal tumours: A new tool to improve the accuracy of nephrometry scores[J]. BJU Int, 2019,124(6):945-954.
doi: 10.1111/bju.14894 |
| [13] |
Tannus M, Goldman SM, Andreoni C. Practical and intuitive surgical approach renal ranking to predict outcomes in the management of renal tumors: A novel score tool[J]. J Endourol, 2014,28(4):487-492.
doi: 10.1089/end.2013.0148 |
| [14] | Nisen H, Ruutu M, Glucker E, et al. Renal tumour invasion index as a novel anatomical classification predicting urological complications after partial nephrectomy[J]. Scand J Urol, 2014,48(1):41-51. |
| [15] |
Leslie S, Gill IS, de Castro AA, et al. Renal tumor contact surface area: A novel parameter for predicting complexity and outcomes of partial nephrectomy[J]. Eur Urol, 2014,66(5):884-893.
doi: 10.1016/j.eururo.2014.03.010 |
| [16] |
Hsieh PF, Wang YD, Huang CP, et al. A mathematical method to calculate tumor contact surface area: An effective parameter to predict renal function after partial nephrectomy[J]. J Urol, 2016,196(1):33-40.
doi: 10.1016/j.juro.2016.01.092 |
| [17] |
Takagi T, Yoshida K, Kondo T, et al. Association between tumor contact surface area and parenchymal volume change in robot-assisted laparoscopic partial nephrectomy carried out using the enucleation technique[J]. Int J Urol, 2019,26(7):745-751.
doi: 10.1111/iju.2019.26.issue-7 |
| [18] |
Kiziloz H, Dorin R, Finnegan KT, et al. The impact of body mass index on perioperative outcomes in robot-assisted laparoscopic partial nephrectomy[J]. J Endourol, 2013,27(8):1000-1007.
doi: 10.1089/end.2012.0665 |
| [19] |
Gong EM, Orvieto MA, Lyon MB, et al. Analysis of impact of body mass index on outcomes of laparoscopic renal surgery[J]. Urology, 2007,69(1):38-43.
doi: 10.1016/j.urology.2006.09.020 |
| [20] |
Macleod LC, Hsi RS, Gore JL, et al. Perinephric fat thickness is an independent predictor of operative complexity during robot-assisted partial nephrectomy[J]. J Endourol, 2014,28(5):587-591.
doi: 10.1089/end.2013.0647 |
| [1] | Kewei CHEN,Zhuo LIU,Shaohui DENG,Fan ZHANG,Jianfei YE,Guoliang WANG,Shudong ZHANG. Clinical diagnosis and treatment of renal angiomyolipoma with inferior vena cava tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 617-623. |
| [2] | Jie YANG,Jieli FENG,Shudong ZHANG,Lulin MA,Qing ZHENG. Clinical effects of transesophageal echocardiography in different surgical methods for nephrectomy combined with Mayo Ⅲ-Ⅳ vena tumor thrombectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 631-635. |
| [3] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [4] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [5] | Min QIU,You-long ZONG,Bin-shuai WANG,Bin YANG,Chu-xiao XU,Zheng-hui SUN,Min LU,Lei ZHAO,Jian LU,Cheng LIU,Xiao-jun TIAN,Lu-lin MA. Treatment outcome of laparoscopic partial nephrectomy in patients with renal tumors of moderate to high complexity [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 833-837. |
| [6] | Quan ZHANG,Hai-feng SONG,Bing-lei MA,Zhe-nan ZHANG,Chao-hui ZHOU,Ao-lin LI,Jun LIU,Lei LIANG,Shi-yu ZHU,Qian ZHANG. Pre-operative prognostic nutritional index as a predictive factor for prognosis in non-metastatic renal cell carcinoma treated with surgery [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 149-155. |
| [7] | HAN Song-chen,HUANG Zi-xiong,LIU Hui-xin,XU Tao. Renal functional compensation after unilateral radical nephrectomy of renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 680-685. |
| [8] | QIU Min,WANG Lu,DENG Shao-hui,TA Peng-fei,GUO Wei,LU Jian,LIU Cheng,MA Lu-lin. Primary application of Gerota’s fascia suspension device in retroperitoneal laparoscopic partial nephrectomy [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 789-792. |
| [9] | Shu-dong ZHANG,Peng HONG,Bin-shuai WANG,Shao-hui DENG,Fan ZHANG,Li-yuan TAO,Cai-guang CAO,Zhen-hua HU,Lu-lin MA. Usefulness of the indocyanine green fluorescence imaging technique in laparoscopic partial nephrectomy [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 657-662. |
| [10] | Xiao-jun TIAN,Min QIU,Zhuo LIU,Ruo-tao XIAO,Yi HUANG,Guo-liang WANG,Xiao-fei HOU,Shu-dong ZHANG,Shen-rong ZHUANG,Lu-lin MA. Single-center study of laparoscopic radical nephrectomy with Mayo 0-2 level inferior vena cava thrombectomy [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1053-1056. |
| [11] | XU Ben, ZHANG Zhe-nan, LUO Cheng, SONG Hai-feng, ZHANG Qian. Comparison of safety and effectiveness between retroperitoneal laparoscopic tumor aspiration and laparoscopic partial nephrectomy in the treatment of renal angiomyolipoma [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 700-704. |
| [12] | HUANG Zi-xiong, ZHANG Xiao-peng, DONG Sen, LIU Shi-jun, YANG Rong-li, ZHOU Yu-shi, MA Wei-guo. Renal mucinous tubular and spindle cell carcinoma combined with multiple bone metastasis: a case report and literature review [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 732-735. |
| [13] | YE Jian-fei, MA Lu-lin, ZHAO Lei, WANG Guo-liang. Segmental vena cava resection for the treatment of renal tumor with invading tumor thrombus [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 183-187. |
| [14] | LIU Chang, CUI Li-gang, WANG Hong-lei. Renal Ewing’s sarcoma/primitive neuroectodermal tumor: a case report and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 919-923. |
| [15] | ZHAO Lei, MA Lu-lin, ZHANG Hong-xian, HOU Xiao-fei, LIU Lei, FU Yan, KOU Yun-geng, SONG Yi-meng. Technical improvement in retroperitoneal laparoscopic living donor nephrectomy: report of 193 cases [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 867-871. |
|
||