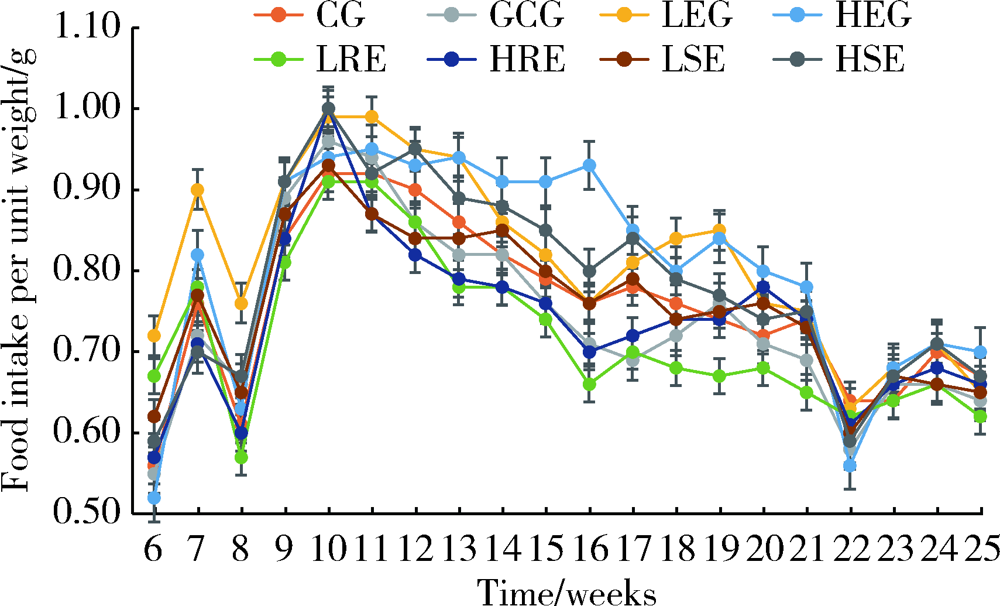
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (2): 244-248. doi: 10.19723/j.issn.1671-167X.2022.02.007
Previous Articles Next Articles
Equol and its enantiomers inhibited urethane-induced lung cancer in mice
YU Xue,ZOU Yong-qiu,WANG Ying,CHEN Ze-kun,MA De-fu( )
)
- Department of Social Medicine and Health Education, Peking University School of Public Health, Beijing 100191, China
CLC Number:
- R73-35+4
| [1] |
Herbst RS, Heymach JV, Lippman SM. Lung cancer[J]. N Engl J Med, 2008, 359(13):1367-1380.
doi: 10.1056/NEJMra0802714 |
| [2] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1):7-30.
doi: 10.3322/caac.v70.1 |
| [3] | 陈万青, 张思维, 邹小农. 中国肺癌发病死亡的估计和流行趋势研究[J]. 中国肺癌杂志, 2010, 13(5):488-493. |
| [4] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2):115-132.
doi: 10.3322/caac.21338 |
| [5] |
Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC)[J]. Respirology, 2015, 20(3):370-378.
doi: 10.1111/resp.12490 pmid: 25689095 |
| [6] |
Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019[J]. CA Cancer J Clin, 2019, 69(5):363-385.
doi: 10.3322/caac.v69.5 |
| [7] |
Shi J, Zhu J. Health resource utilization in patients with advanced non-small cell lung cancer receiving chemotherapy in China[J]. Clin Drug Investig, 2016, 36(1):77-86.
doi: 10.1007/s40261-015-0356-9 |
| [8] |
Zhong L, Yang J, Cao Z, et al. Preclinical pharmacodynamic evaluation of drug candidate SKLB-178 in the treatment of non-small cell lung cancer[J]. Oncotarget, 2017, 8(8):12843-13854.
doi: 10.18632/oncotarget.14597 pmid: 28086226 |
| [9] | Norat T, Aune D, Chan D, et al. Fruits and vegetables: Updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention[J]. Cancer Treat Res, 2014, 159:35-50. |
| [10] | Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta[J]. Bioorg Med Chem, 2004, 12(6):1559-1567. |
| [11] |
Brown NM, Belles CA, Lindley SL, et al. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer[J]. Carcinogenesis, 2010, 31(5):886-893.
doi: 10.1093/carcin/bgq025 |
| [12] |
Zhou T, Meng C, He P. Soy Isoflavones and their effects on xenobiotic metabolism[J]. Curr Drug Metab, 2019, 20(1):46-53.
doi: 10.2174/1389200219666180427170213 |
| [13] |
Ma D, Zhang Y, Yang T, et al. Isoflavone intake inhibits the development of 7,12-dimethylbenz(a)anthracene(DMBA)-induced mammary tumors in normal and ovariectomized rats[J]. J Clin Biochem Nutr, 2014, 54(1):31-38.
doi: 10.3164/jcbn.13-33 |
| [14] |
Mayo B, Vázquez L, Flórez AB. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects[J]. Nutrients, 2019, 11(9):2231.
doi: 10.3390/nu11092231 |
| [15] |
Lignitto L, LeBoeuf SE, Homer H, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of bach1[J]. Cell, 2019, 178(2): 316.e18-329. e18.
doi: S0092-8674(19)30631-2 pmid: 31257023 |
| [16] |
Mohs A, Otto T, Schneider KM, et al. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis[J]. J Hepatol, 2021, 74(3):638-648.
doi: 10.1016/j.jhep.2020.09.037 |
| [1] | Qiao ZHU,Cui REN,Yan ZHANG,Mei-jiao LI,Xiao-hua WANG. Comparative imaging study of mediastinal lymph node from pre-surgery dual energy CT versus post-surgeron verifications in non-small cell lung cancer patients [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 730-737. |
| [2] | Yu-qing OUYANG,Lian-fang NI,Xin-min LIU. Prognosis factors analysis of patients with malignant solitary pulmonary nodules [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 158-162. |
| [3] | CAI Yuan-fa, ZHANG Hua-ming, NIU Wen-yi, ZOU Yong-qiu, MA De-fu. Effects of equol on colon cancer cell proliferation [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 383-387. |
|
||