Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 228-233. doi: 10.19723/j.issn.1671-167X.2023.02.005
Previous Articles Next Articles
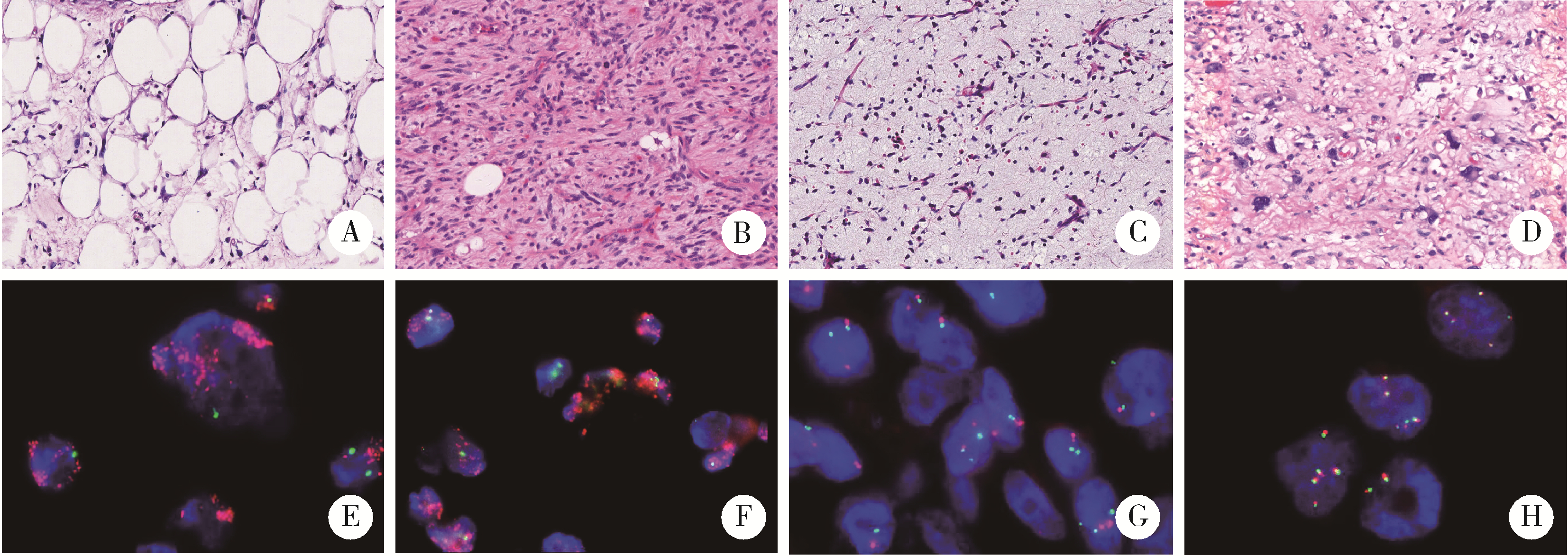
Clinical value of fluorescence in situ hybridization with MDM2 and DDIT3 probe in diagnosis of liposarcoma
Wei WANG,Xin LI,Ping LIU,Ying DONG*( )
)
- Department of Pathology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R365
| 1 | WHO Classification of Tumours Editorial Board . WHO classification of tumours of soft tissue and bone[M]. 5th ed Lyon, France: IARC Press, 2020: 34- 46. |
| 2 | Nishio J . Contributions of cytogenetics and molecular cytogenetics to the diagnosis of adipocytic tumors[J]. J Biomed Biotechnol, 2011, 2011, 524067. |
| 3 |
Weaver J , Rao P , Goldblum JR , et al. Can MDM2 analytical tests performed on core needle biopsy be relied upon to diagnose well-differentiated liposarcoma?[J]. Mod Pathol, 2010, 23 (10): 1301- 1306.
doi: 10.1038/modpathol.2010.106 |
| 4 |
Mantilla JG , Ricciotti RW , Chen EY , et al. Amplifification of DNA damage-inducible transcript 3 (DDIT3) is associated with myxoid liposarcoma-like morphology and homologous lipoblastic differentiation in dedifferentiated liposarcoma[J]. Mod Pathol, 2019, 32 (4): 585- 592.
doi: 10.1038/s41379-018-0171-y |
| 5 |
Kuczkiewicz-Siemion O , Wiśniewski P , Dansonka-Mieszkowska A , et al. The utility of fluorescence in situ hybridization (FISH) in determining DNA damage-inducible transcript 3 (DDIT3) amplification in dedifferentiated liposarcomas-an important diagnostic pitfall[J]. Pathol Res Pract, 2021, 225, 153555.
doi: 10.1016/j.prp.2021.153555 |
| 6 |
Cho J , Lee SE , Choi YL . Diagnostic value of MDM2 and DDIT3 fluorescence in situ hybridization in liposarcoma classification: A single-institution experience[J]. Korean J Pathol, 2012, 46 (2): 115- 122.
doi: 10.4132/KoreanJPathol.2012.46.2.115 |
| 7 |
Vargas AC , Selinger C , Satgunaseelan L , et al. FISH analysis of selected soft tissue tumors: Diagnostic experience in a tertiary center[J]. Asia Pac J Clin Oncol, 2019, 15 (1): 38- 47.
doi: 10.1111/ajco.12980 |
| 8 |
Sugita S , Seki K , Yokozawa K , et al. Analysis of CHOP rearrangement in pleomorphic liposarcomas using fluorescence in situ hybridization[J]. Cancer Sci, 2009, 100 (1): 82- 87.
doi: 10.1111/j.1349-7006.2008.01008.x |
| 9 | Creytens D , van Gorp J , Speel EJ , et al. Characterization of the 12q amplicons in lipomatous soft tissue tumors by multiplex ligation-dependent probe amplification-based copy number analysis[J]. Anticancer Res, 2015, 35 (4): 1835- 1842. |
| 10 |
Demicco EG . Molecular updates in adipocytic neoplasms[J]. Semin Diagn Pathol, 2019, 36 (2): 85- 94.
doi: 10.1053/j.semdp.2019.02.003 |
| 11 | 杨邵敏, 吴若晨, 齐双双, 等. 睾丸旁脂肪肉瘤19例临床病理学特征[J]. 中华病理学杂志, 2022, 51 (1): 17- 22. |
| 12 |
Jauhiainen A , Thomsen C , Strömbom L , et al. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153[J]. PLoS One, 2012, 7 (4): e33208.
doi: 10.1371/journal.pone.0033208 |
| 13 |
Yu JSE , Colborne S , Hughes CS , et al. The FUS-DDIT3 interactome in myxoid liposarcoma[J]. Neoplasia, 2019, 21 (8): 740- 751.
doi: 10.1016/j.neo.2019.05.004 |
| 14 |
Berthold R , Isfort I , Erkut C , et al. Fusion protein-driven IGF-IR/PI3K/AKT signals deregulate Hippo pathway promoting oncogenic cooperation of YAP1 and FUS-DDIT3 in myxoid liposarcoma[J]. Oncogenesis, 2022, 11 (1): 20.
doi: 10.1038/s41389-022-00394-7 |
| 15 |
李锐, 叶胜兵, 赵明, 等. 脂肪肉瘤中DDIT3荧光原位杂交分离探针判读陷阱及其意义分析[J]. 中华病理学杂志, 2022, 51 (3): 230- 233.
doi: 10.3760/cma.j.cn112151-20210609-00426 |
| 16 |
Murshed KA , Abo-Samra H , Ammar A . Well-differentiated liposarcoma of the hypopharynx exhibiting myxoid liposarcoma-like morphology with MDM2 and DDIT3 co-amplification[J]. Head Neck Pathol, 2022, 16 (1): 288- 293.
doi: 10.1007/s12105-021-01341-5 |
| 17 |
Sioletic S , Paola DC , Fletcher CD , et al. Well-differentiated and dedifferentiated liposarcomas with prominent myxoid stroma: Ana-lysis of 56 cases[J]. Histopathology, 2013, 62 (2): 287- 293.
doi: 10.1111/j.1365-2559.2012.04348.x |
| 18 |
Rao UN , Cieply K , Sherer C , et al. Correlation of classic and mole-cular cytogenetic alterations in soft-tissue sarcomas: Analysis of 46 tumors with emphasis on adipocytic tumors and synovial sarcoma[J]. Appl Immunohistochem Mol Morphol, 2017, 25 (3): 168- 177.
doi: 10.1097/PAI.0000000000000294 |
| [1] | 挺 李. [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 197-200. |
| [2] | 桥 周. [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 201-209. |
| [3] | Yun-yi XU,Zheng-zheng SU,Lin-mao ZHENG,Meng-ni ZHANG,Jun-ya TAN,Ya-lan YANG,Meng-xin ZHANG,Miao XU,Ni CHEN,Xue-qin CHEN,Qiao ZHOU. Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 217-227. |
| [4] | Dong LI,Ji-ting DI,Yan XIONG. Consistency comparison of programmed cell death 1-ligand 1 in different immuno-histochemical staining methods [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 339-342. |
| [5] | Yan XIONG,Bo ZHANG,Li-gong NIE,Shi-kai WU,Hu ZHAO,Dong LI,Ji-ting DI. Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 351-356. |
| [6] | CHEN Zhi-qiang, WANG Ying, MI Xian-jun, CHEN Ang, HUANG Hua-yong, ZHONG Shou-jun, DENG Wen-tong, LIU Chao-fan, XU Xiu-mei, DAI Xin-zhen. Comparison between poly hydroxy acrylic acid and Van-clear replacing the tradi-tional reagents to detect the cervical hTERC genes by adopting FISH technique [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 356-360. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 209
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 752
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||