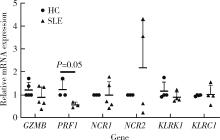
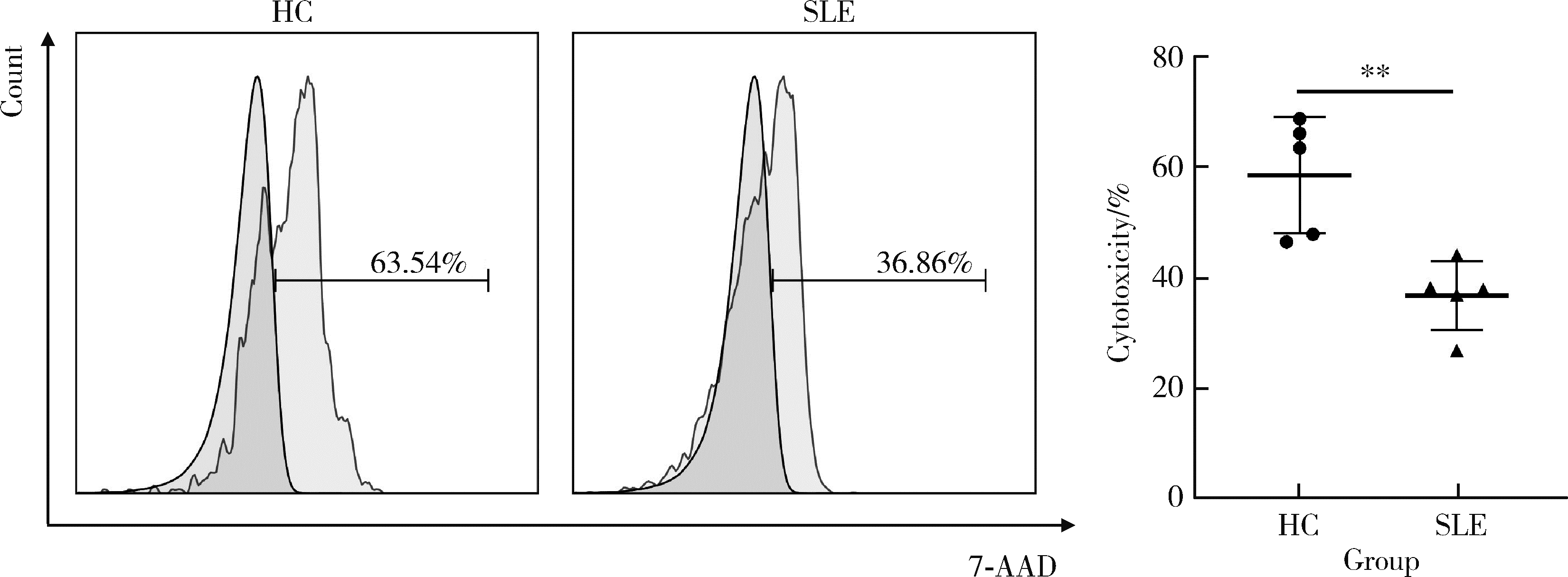
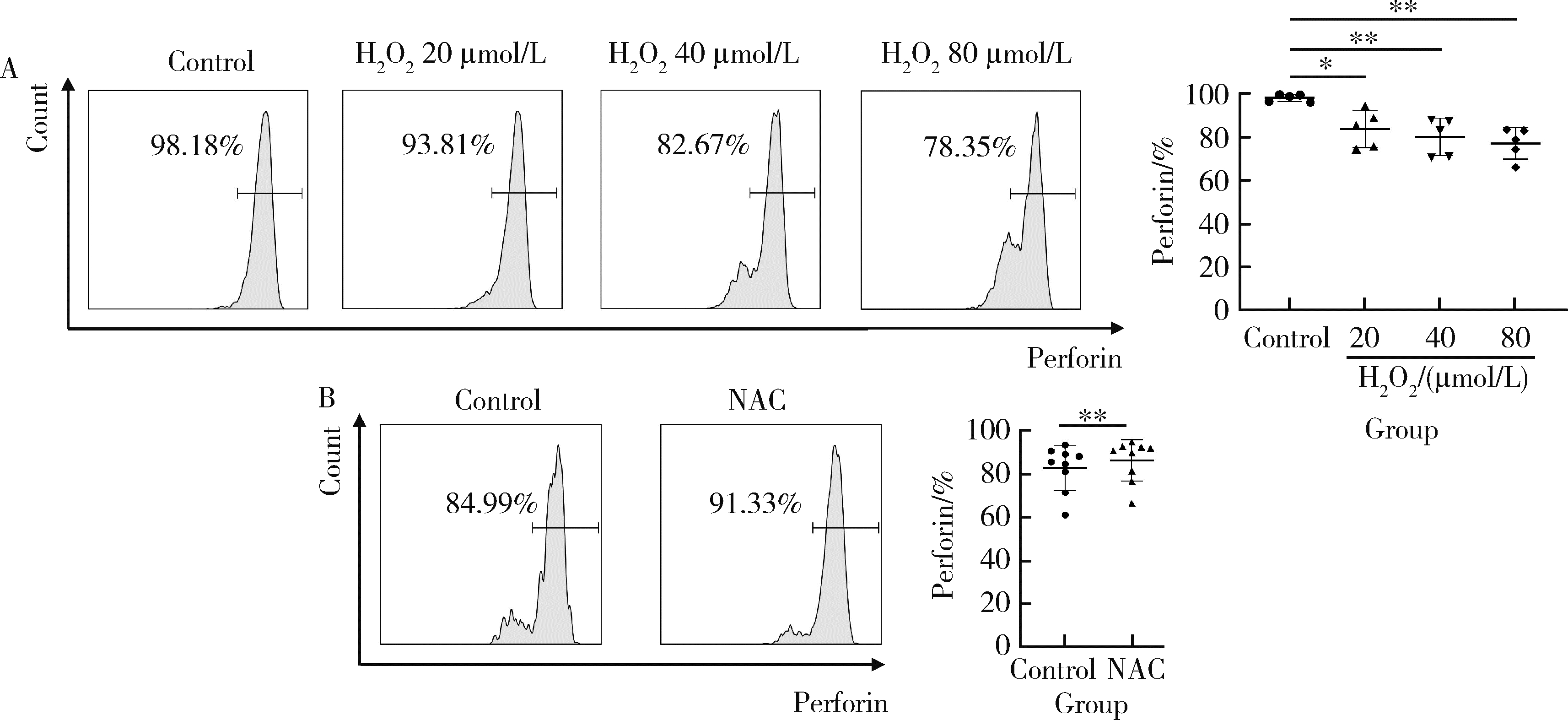
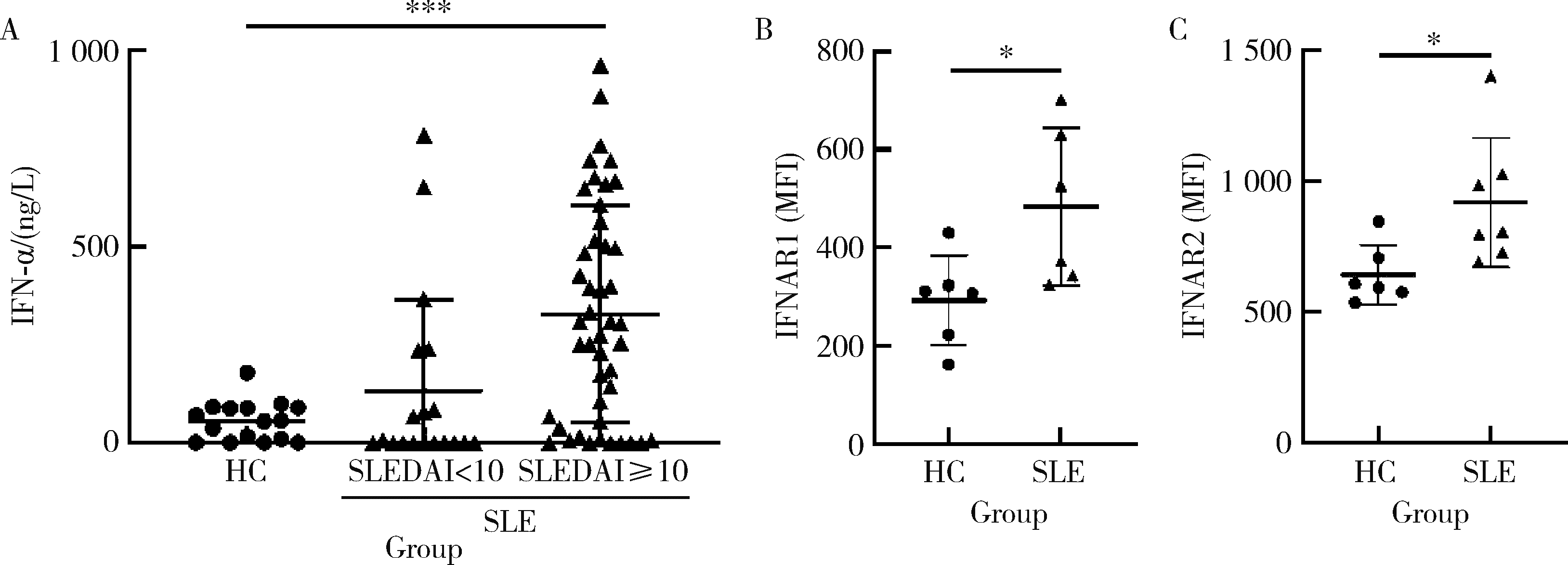
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (6): 975-981. doi: 10.19723/j.issn.1671-167X.2023.06.004
Previous Articles Next Articles
Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss
Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI*( )
)
- Department of Immunology, College of Basic Medical Science, Dalian Medical University, Dalian 116044, Liaoning, China
CLC Number:
- R593.2
| 1 |
Humbel M , Bellanger F , Fluder N , et al. Restoration of NK cell cytotoxic function with elotuzumab and daratumumab promotes elimination of circulating plasma cells in patients with SLE[J]. Front Immunol, 2021, 12, 645478.
doi: 10.3389/fimmu.2021.645478 |
| 2 |
Cheng Q , Chen X , Xu J , et al. lncRNA X-inactive-specific transcript is a potential biomarker related to changes in CD4+T cell levels in systemic lupus erythematosus[J]. Rheumatol Autoimmun, 2022, 2 (3): 159- 174.
doi: 10.1002/rai2.12048 |
| 3 | Lin SJ , Kuo ML , Hsiao HS , et al. Cytotoxic function and cytokine production of natural killer cells and natural killer T-like cells in systemic lupus erythematosis regulation with interleukin-15[J]. Mediators Inflamm, 2019, 2019, 4236562. |
| 4 |
Sordo-Bahamonde C , Lorenzo-Herrero S , Payer ÁR , et al. Mechanisms of apoptosis resistance to NK cell-mediated cytotoxicity in cancer[J]. Int J Mol Sci, 2020, 21 (10): 3726.
doi: 10.3390/ijms21103726 |
| 5 | Lorenzo-Herrero S , Sordo-Bahamonde C , Gonzalez S , et al. CD107a degranulation assay to evaluate immune cell antitumor activity[J]. Methods Mol Biol, 2019, 1884, 119- 130. |
| 6 |
Myers JA , Miller JS . Exploring the NK cell platform for cancer immunotherapy[J]. Nat Rev Clin Oncol, 2021, 18 (2): 85- 100.
doi: 10.1038/s41571-020-0426-7 |
| 7 |
Quatrini L , Della Chiesa M , Sivori S , et al. Human NK cells, their receptors and function[J]. Eur J Immunol, 2021, 51 (7): 1566- 1579.
doi: 10.1002/eji.202049028 |
| 8 |
Kucuksezer UC , Aktas Cetin E , Esen F , et al. The role of natural killer cells in autoimmune diseases[J]. Front Immunol, 2021, 12, 622306.
doi: 10.3389/fimmu.2021.622306 |
| 9 |
Bryceson YT , March ME , Barber DF , et al. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells[J]. J Exp Med, 2005, 202 (7): 1001- 1012.
doi: 10.1084/jem.20051143 |
| 10 | Nielsen CM , White MJ , Goodier MR , et al. Functional significance of CD57 expression on human NK cells and relevance to disease[J]. Front Immunol, 2013, 4, 422. |
| 11 |
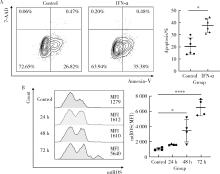
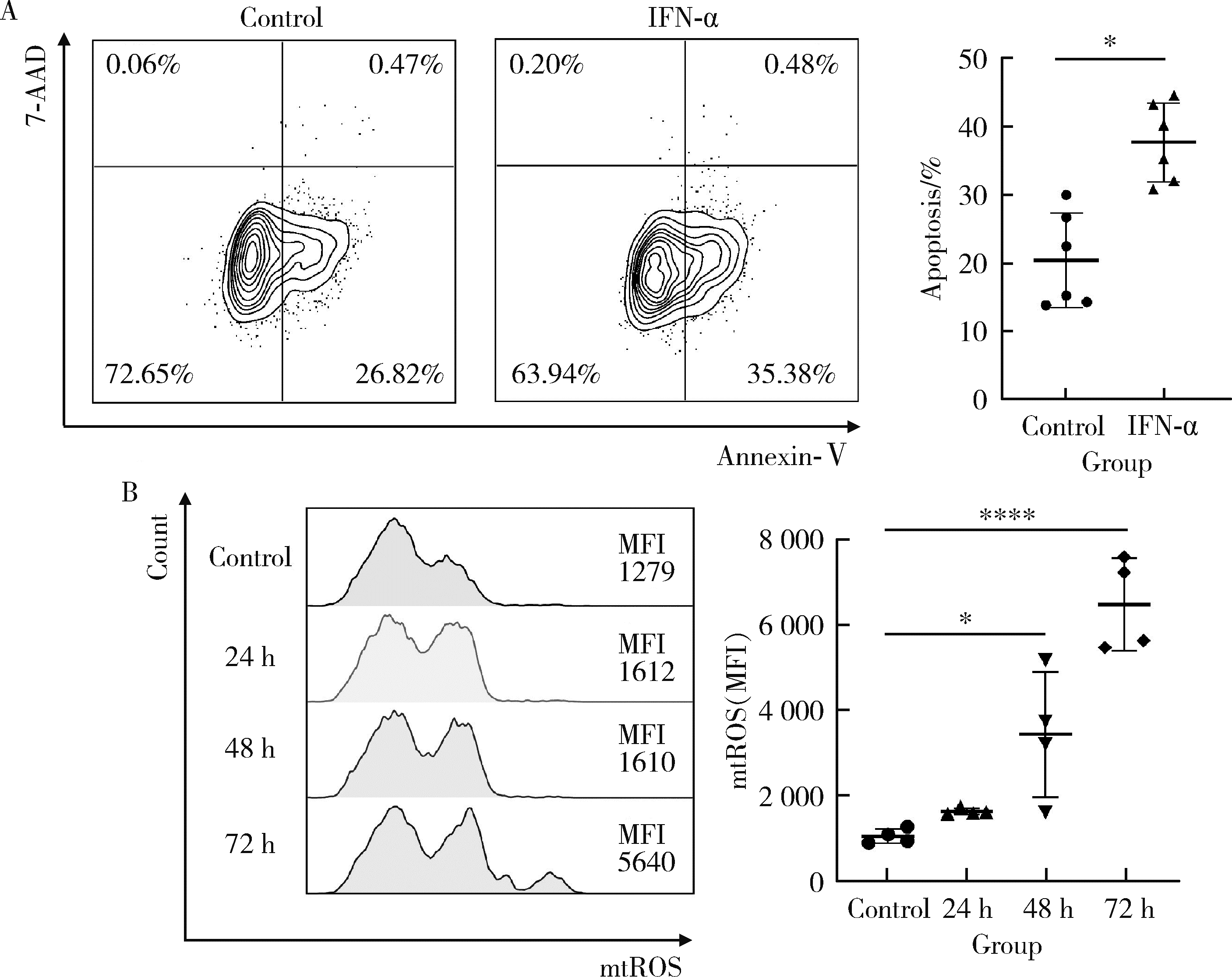
Lu Z , Tian Y , Bai Z , et al. Increased oxidative stress contributes to impaired peripheral CD56dimCD57+ NK cells from patients with systemic lupus erythematosus[J]. Arthritis Res Ther, 2022, 24 (1): 48.
doi: 10.1186/s13075-022-02731-y |
| 12 |
Banchereau R , Hong S , Cantarel B , et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients[J]. Cell, 2016, 165 (3): 551- 565.
doi: 10.1016/j.cell.2016.03.008 |
| 13 |
Li P , Jiang M , Li K , et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity[J]. Nat Immunol, 2021, 22 (9): 1107- 1117.
doi: 10.1038/s41590-021-00993-3 |
| 14 |
Furie R , Werth VP , Merola JF , et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus[J]. J Clin Invest, 2019, 129 (3): 1359- 1371.
doi: 10.1172/JCI124466 |
| 15 |
Chatham WW , Furie R , Saxena A , et al. Long-term safety and efficacy of anifrolumab in adults with systemic lupus erythematosus: Results of a phase Ⅱ open-label extension study[J]. Arthritis Rheumatol, 2021, 73 (5): 816- 825.
doi: 10.1002/art.41598 |
| 16 |
Kirou KA , Mavragani CP , Crow MK . Activation of type I inter-feron in systemic lupus erythematosus[J]. Expert Rev Clin Immunol, 2007, 3 (4): 579- 588.
doi: 10.1586/1744666X.3.4.579 |
| 17 |
Le Bon A , Thompson C , Kamphuis E , et al. Cutting edge: Enhancement of antibody responses through direct stimulation of B and T cells by type I IFN[J]. J Immunol, 2006, 176 (4): 2074- 2078.
doi: 10.4049/jimmunol.176.4.2074 |
| 18 |
Cucak H , Yrlid U , Reizis B , et al. Type Ⅰ interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells[J]. Immunity, 2009, 31 (3): 491- 501.
doi: 10.1016/j.immuni.2009.07.005 |
| 19 | De Groof A , Ducreux J , Aleva F , et al. STAT3 phosphorylation mediates the stimulatory effects of interferon alpha on B cell dif-ferentiation and activation in SLE[J]. Rheumatology (Oxford), 2020, 59 (3): 668- 677. |
| 20 | Hochberg MC . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40 (9): 1725. |
| 21 | Li H , Geng L , Cao Z , et al. CD56brightCD16- to CD57+CD56dimCD16+ NK cell ratio discriminates disease activity and renal involvement in patients with systemic lupus erythematosus[J]. Clin Exp Rheumatol, 2023, 41 (9): 1768- 1776. |
| 22 |
Yang Y , Day J , Souza-Fonseca Guimaraes F , et al. Natural killer cells in inflammatory autoimmune diseases[J]. Clin Transl Immunology, 2021, 10 (2): e1250.
doi: 10.1002/cti2.1250 |
| 23 |
Streltsova MA , Erokhina SA , Kanevskiy LM , et al. Analysis of NK cell clones obtained using interleukin-2 and gene-modified K562 cells revealed the ability of "senescent" NK cells to lose CD57 expression and start expressing NKG2A[J]. PLoS One, 2018, 13 (12): e0208469.
doi: 10.1371/journal.pone.0208469 |
| 24 | Bahadorian D , Mollazadeh S , Mirazi H , et al. Regulatory NK cells in autoimmune disease[J]. Iran J Basic Med Sci, 2023, 26 (6): 609- 616. |
| 25 |
Fresneda Alarcon M , McLaren Z , Wright HL . Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O[J]. Front Immunol, 2021, 12, 649693.
doi: 10.3389/fimmu.2021.649693 |
| 26 |
Shah D , Mahajan N , Sah S , et al. Oxidative stress and its biomarkers in systemic lupus erythematosus[J]. J Biomed Sci, 2014, 21 (1): 23.
doi: 10.1186/1423-0127-21-23 |
| 27 |
Kennel KB , Greten FR . Immune cell-produced ROS and their impact on tumor growth and metastasis[J]. Redox Biol, 2021, 42, 101891.
doi: 10.1016/j.redox.2021.101891 |
| 28 |
Song H , Park H , Kim YS , et al. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species[J]. Int Immunopharmacol, 2011, 11 (8): 932- 938.
doi: 10.1016/j.intimp.2011.02.005 |
| 29 |
He H , Wang C , Liu G , et al. Isobavachalcone inhibits acute myeloid leukemia: Potential role for ROS-dependent mitochondrial apoptosis and differentiation[J]. Phytother Res, 2021, 35 (6): 3337- 3350.
doi: 10.1002/ptr.7054 |
| 30 |
Bhattacharya A , Hegazy AN , Deigendesch N , et al. Superoxide dismutase 1 protects hepatocytes from type I interferon-driven oxidative damage[J]. Immunity, 2015, 43 (5): 974- 986.
doi: 10.1016/j.immuni.2015.10.013 |
| 31 |
Tasdogan A , Kumar S , Allies G , et al. DNA damage-induced HSPC malfunction depends on ROS accumulation downstream of IFN-1 signaling and bid mobilization[J]. Cell Stem Cell, 2016, 19 (6): 752- 767.
doi: 10.1016/j.stem.2016.08.007 |
| 32 |
Buang N , Tapeng L , Gray V , et al. Type Ⅰ interferons affect the metabolic fitness of CD8+ T cells from patients with systemic lupus erythematosus[J]. Nat Commun, 2021, 12 (1): 980.
doi: 10.1038/s41467-021-21210-7 |
| [1] | Jiayi TIAN, Yixue GUO, Xia ZHANG, Xiaolin SUN, Jing HE. Flow cytometry analysis of normal range of natural killer cells and their subsets in peripheral blood of healthy Chinese adults [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 839-844. |
| [2] | Zhihui WU, Mingzhi HU, Qiaoying ZHAO, Fengfeng LV, Jingying ZHANG, Wei ZHANG, Yongfu WANG, Xiaolin SUN, Hui WANG. Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 860-867. |
| [3] | Limin REN,Chuchu ZHAO,Yi ZHAO,Huiqiong ZHOU,Liyun ZHANG,Youlian WANG,Lingxun SHEN,Wenqiang FAN,Yang LI,Xiaomei LI,Jibo WANG,Yongjing CHENG,Jiajing PENG,Xiaozhen ZHAO,Miao SHAO,Ru Li. Low disease activity and remission status of systemic lupus erythematosus in a real-world study [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 273-278. |
| [4] | Zhi-jun LUO,Jia-jia WU,You SONG,Chun-li MEI,Rong DU. Systemic lupus erythematosus associated macrophage activation syndrome with neuropsychiatric symptoms: A report of 2 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1111-1117. |
| [5] | Hai-hong YAO,Fan YANG,Su-mei TANG,Xia ZHANG,Jing HE,Yuan JIA. Clinical characteristics and diagnostic indicators of macrophage activation syndrome in patients with systemic lupus erythematosus and adult-onset Still's disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 966-974. |
| [6] | Lin-qi ZHANG,Jing ZHAO,Hong-yan WANG,Zong-yi WANG,Ying-ni LI,Ji-yang TANG,Si-ying LI,Jin-feng QU,Ming-wei ZHAO. Relationship between anti-ENO1 antibody and systemic lupus erythematosus patients with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1099-1105. |
| [7] | Min LI,Lin-qing HOU,Yue-bo JIN,Jing HE. Clinical and immunological characteristics of systemic lupus erythematosus with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1106-1111. |
| [8] | Miao SHAO,Hui-fang GUO,Ling-yan LEI,Qing ZHAO,Yan-jie DING,Jin LIN,Rui WU,Feng YU,Yu-cui LI,Hua-li MIAO,Li-yun ZHANG,Yan DU,Rui-ying JIAO,Li-xia PANG,Li LONG,Zhan-guo LI,Ru LI. A multicenter study on the tolerance of intravenous low-dose cyclophosphamide in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1112-1116. |
| [9] | Xiu-rui LIANG,Xue-chun SHAN,Jing GUAN,Rui ZHANG,Jing YANG,Yi ZHANG,Jia-qi JIN,Yu-xin ZHANG,Fan XU,Ji-hua FU. Role of hyperglycemia-induced 5-hydroxytryptamine degradation of hepatic stellate cells in hepatic inflammation and fibrosis induced by type 2 diabetes mellitus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1141-1150. |
| [10] | Jian-mei ZOU,Li-jun WU,Cai-nan LUO,Ya-mei SHI,Xue WU. Relationship of serum 25- hydroxy vitamin D and systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 938-941. |
| [11] | MA Xiang-bo,ZHANG Xue-wu,JIA Ru-lin,GAO Ying,LIU Hong-jiang,LIU Yu-fang,LI Ying-ni. Application of lymphocytes test in peripheral blood of patients with systemic sclerosis during the treatment [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 721-727. |
| [12] | XIA Fang-fang,LU Fu-ai,LV Hui-min,YANG Guo-an,LIU Yuan. Clinical characteristics and related factors of systemic lupus erythematosus with interstitial pneumonia [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 266-272. |
| [13] | Dong YAN,Wen-jie ZHENG. Progress in interferon: A treatment of Behcet syndrome [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1166-1170. |
| [14] | Yan GENG,Bo-rui LI,Zhuo-li ZHANG. Musculoskeletal ultrasound findings of symptomatic joints in patients with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 163-168. |
| [15] | Yu-hua WANG,Guo-hua ZHANG,Ling-ling ZHANG,Jun-li LUO,Lan GAO. Adrenal hemorrhage in a patient with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1178-1181. |
|
||