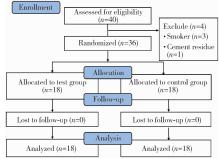
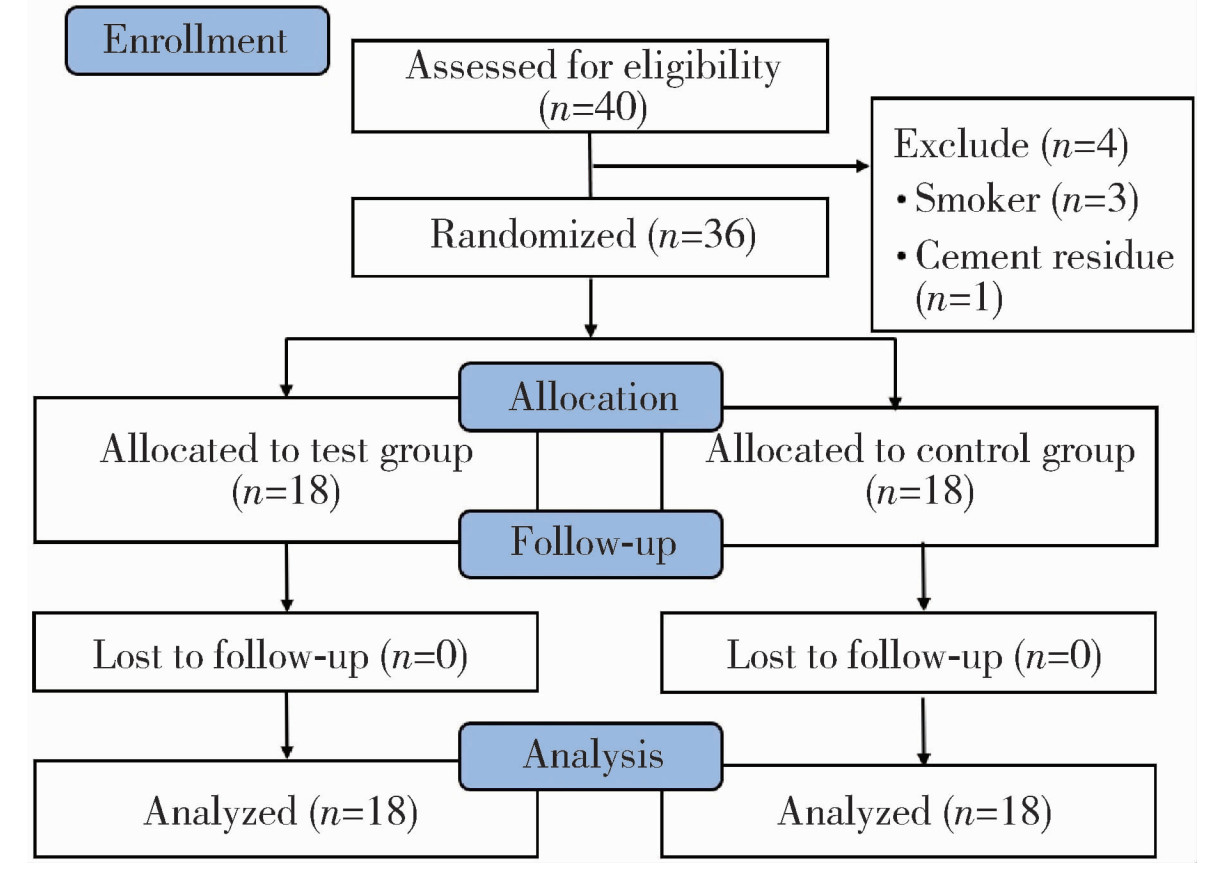
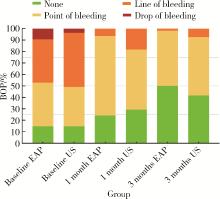
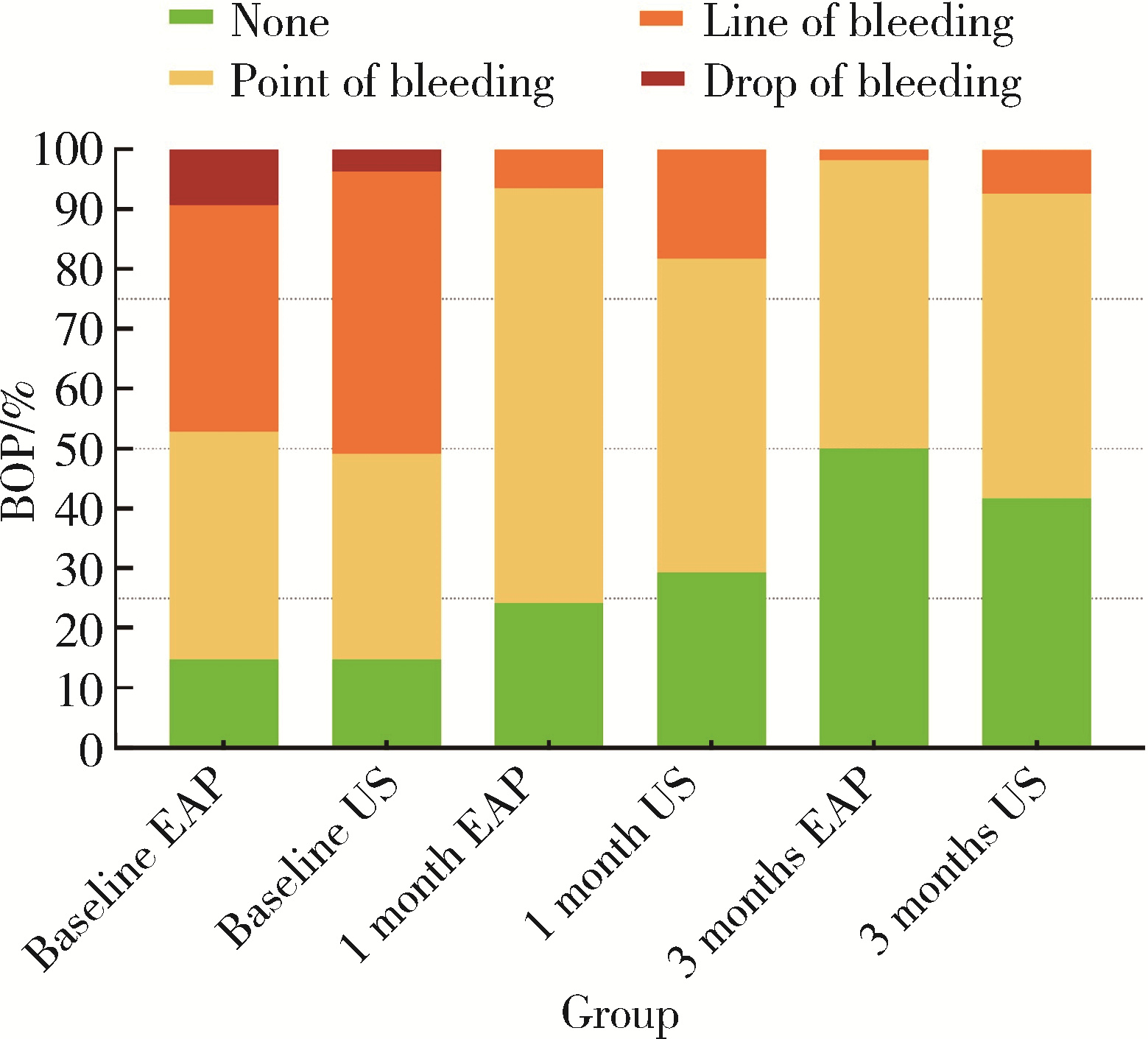
| 1 |
Dos Reis INR, Huamán-Mendoza AA, Ramadan D, et al. The prevalence of peri-implant mucositis and peri-implantitis based on the world workshop criteria: A systematic review and meta-analysis[J]. J Dent, 2025, 160, 105914.
doi: 10.1016/j.jdent.2025.105914
|
| 2 |
Romandini M, Lima C, Pedrinaci I, et al. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study[J]. Clin Oral Implants Res, 2021, 32(1): 112- 122.
doi: 10.1111/clr.13684
|
| 3 |
Djuran B, Tatic Z, Perunovic N, et al. Underdiagnosis in background of emerging public health challenges related to peri-implant diseases: An interventional split-mouth study[J]. Int J Environ Res Public Health, 2022, 20(1): 477.
doi: 10.3390/ijerph20010477
|
| 4 |
Galarraga-Vinueza ME, Pagni S, Finkelman M, et al. Prevalence, incidence, systemic, behavioral, and patient-related risk factors and indicators for peri-implant diseases: An AO/AAP systematic review and meta-analysis[J]. J Periodontol, 2025, 96(6): 587- 633.
doi: 10.1002/JPER.24-0154
|
| 5 |
Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions[J]. J Clin Periodontol, 2018, 45(Suppl 20): S286- S291.
|
| 6 |
Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans[J]. Clin Oral Implants Res, 2012, 23(2): 182- 190.
doi: 10.1111/j.1600-0501.2011.02220.x
|
| 7 |
Meyer S, Giannopoulou C, Courvoisier D, et al. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses[J]. Clin Oral Implants Res, 2017, 28(8): 1005- 1012.
doi: 10.1111/clr.12912
|
| 8 |
Menini M, Delucchi F, Bagnasco F, et al. Efficacy of air-polishing devices without removal of implant-supported full-arch prostheses[J]. Int J Oral Implantol, 2021, 14(4): 401- 416.
|
| 9 |
Tan SL, Grewal GK, Mohamed Nazari NS, et al. Efficacy of air polishing in comparison with hand instruments and/or power-driven instruments in supportive periodontal therapy and implant maintenance: A systematic review and meta-analysis[J]. BMC Oral Health, 2022, 22(1): 85.
doi: 10.1186/s12903-022-02120-6
|
| 10 |
Bengtsson VW, Aoki A, Mizutani K, et al. Treatment of peri-implant mucositis using an Er: YAG laser or an ultrasonic device: A randomized, controlled clinical trial[J]. Int J Implant Dent, 2025, 11(1): 6.
doi: 10.1186/s40729-025-00591-0
|
| 11 |
Herrera D, Berglundh T, Schwarz F, et al. Prevention and treatment of peri-implant diseases: The EFP S3 level clinical practice guideline[J]. J Clin Periodontol, 2023, 50(Suppl 26): 4- 76.
|
| 12 |
Lupi SM, Granati M, Butera A, et al. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: A six-month randomized clinical trial[J]. Int J Dent Hyg, 2017, 15(4): 287- 294.
doi: 10.1111/idh.12206
|
| 13 |
Mensi M, Cochis A, Sordillo A, et al. Biofilm removal and bacterial re-colonization inhibition of a novel erythritol/chlorhexidine air-polishing powder on titanium disks[J]. Materials, 2018, 11(9): 1510.
doi: 10.3390/ma11091510
|
| 14 |
Verket A, Koldsland OC, Bunaes D, et al. Non-surgical therapy of peri-implant mucositis-mechanical/physical approaches: A systematic review[J]. J Clin Periodontol, 2023, 50(Suppl 26): S135- S145.
|
| 15 |
Riben-Grundstrom C, Norderyd O, André U, et al. Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device: A randomized clinical trial[J]. J Clin Perio-dontol, 2015, 42(5): 462- 469.
doi: 10.1111/jcpe.12395
|
| 16 |
靖无迪, 王宪娥, 谢也斯, 等. 甘氨酸龈下喷砂治疗早期种植体周围病的疗效观察[J]. 中华口腔医学杂志, 2017, 52(8): 480- 485.
|
| 17 |
Nicola D, Isabella DR, Carolina C, et al. Treatment of peri-implant mucositis: Adjunctive effect of glycine powder air polishing to professional mechanical biofilm removal. 12 months randomized clinical study[J]. Clin Implant Dent Relat Res, 2024, 26(2): 415- 426.
doi: 10.1111/cid.13304
|
| 18 |
Corbella S, Radaelli K, Alberti A, et al. Erythritol powder airflow for the treatment of peri-implant mucositis: A randomized controlled clinical trial[J]. Int J Dent Hyg, 2024, 22(4): 982- 990.
doi: 10.1111/idh.12814
|
| 19 |
Ulvik IM, Sæthre T, Bunæs DF, et al. A 12-month randomized controlled trial evaluating erythritol air-polishing versus curette/ultrasonic debridement of mandibular furcations in supportive periodontal therapy[J]. BMC Oral Health, 2021, 21(1): 38.
doi: 10.1186/s12903-021-01397-3
|
 ), Wenjie HU2,*(
), Wenjie HU2,*( )
)