Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (1): 119-125. doi: 10.19723/j.issn.1671-167X.2022.01.019
Previous Articles Next Articles
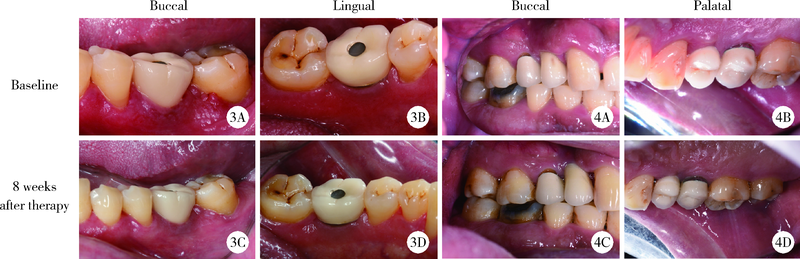
Efficacy of combined application of glycine powder air-polishing in non-surgical treatment of peri-implant diseases
Fei SUN,Si-qi LI,Yi-ping WEI,Jin-sheng ZHONG,Cui WANG( ),Wen-jie HU(
),Wen-jie HU( )
)
- Department of Periodontology, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry, Beijing 100081, China
CLC Number:
- R781.42
| [1] |
Howe M-S, Keys W, Richards D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis[J]. J Dent, 2019, 84:9-21.
doi: 10.1016/j.jdent.2019.03.008 |
| [2] |
Adler L, Buhlin K, Jansson L. Survival and complications: A 9- to 15-year retrospective follow-up of dental implant therapy[J]. J Oral Rehabil, 2020, 47(1):67-77.
doi: 10.1111/joor.v47.1 |
| [3] |
Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology[J]. J Clin Periodontol, 2015, 42(Suppl 16):S158-S171.
doi: 10.1111/jcpe.12334 |
| [4] |
Lupi SM, Granati M, Butera A, et al. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: A six-month randomized clinical trial[J]. Int J Dent Hyg, 2017, 15(4):287-294.
doi: 10.1111/idh.12206 pmid: 26842543 |
| [5] |
Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis[J]. J Clin Periodontol, 2015, 42(Suppl 16):S152-S157.
doi: 10.1111/jcpe.12369 |
| [6] | 靖无迪, 王宪娥, 谢也斯, 等. 甘氨酸龈下喷砂治疗早期种植体周围病的疗效观察[J]. 中华口腔医学杂志, 2017, 52(8):480-485. |
| [7] |
Sahm N, Becker J, Santel T, et al. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study[J]. J Clin Periodontol, 2011, 38(9):872-878.
doi: 10.1111/jcpe.2011.38.issue-9 |
| [8] |
Berglundh T, Armitage G, Araujo M G, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions[J]. J Clin Periodontol, 2018, 45(Suppl 20):S286-S291.
doi: 10.1111/jcpe.2018.45.issue-S20 |
| [9] |
Shrivastava D, Natoli V, Srivastava KC, et al. Novel approach to dental biofilm management through guided biofilm therapy (GBT): A review[J]. Microorganisms, 2021, 9(9):1966.
doi: 10.3390/microorganisms9091966 |
| [10] | Renvert S, Quirynen M. Risk indicators for peri-implantitis. A narrative review[J]. Clin Oral Implants Res, 2015, 26(Suppl 11):15-44. |
| [11] |
Renvert S, Polyzois I. Risk indicators for peri-implant mucositis: A systematic literature review[J]. J Clin Periodontol, 2015, 42(Suppl 16):S172-S186.
doi: 10.1111/jcpe.12346 |
| [12] |
Steiger-Ronay V, Merlini A, Wiedemeier DB, et al. Location of unaccessible implant surface areas during debridement in simulated peri-implantitis therapy[J]. BMC Oral Health, 2017, 17(1):137.
doi: 10.1186/s12903-017-0428-8 pmid: 29183313 |
| [13] | Cha JK, Paeng K, Jung UW, et al. The effect of five mechanical instrumentation protocols on implant surface topography and roughness: A scanning electron microscope and confocal laser scanning microscope analysis[J]. Clin Oral Implants Res, 2019, 30(6):578-587. |
| [14] |
Tong Z, Fu R, Zhu W, et al. Changes in the surface topography and element proportion of clinically failed SLA implants after in vitro debridement by different methods[J]. Clin Oral Implants Res, 2021, 32(3):263-273.
doi: 10.1111/clr.v32.3 |
| [15] | Ronay V, Merlini A, Attin T, et al. In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach[J]. Clin Oral Implants Res, 2017, 28(2):151-155. |
| [16] |
Schwarz F, Becker K, Renvert S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: A systematic review[J]. J Clin Periodontol, 2015, 42(10):951-959.
doi: 10.1111/jcpe.12454 pmid: 26362615 |
| [17] | Ji YJ, Tang ZH, Wang R, et al. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: A pilot clinical trial[J]. Clin Oral Implants Res, 2014, 25(6):683-689. |
| [18] |
Iatrou P, Chamilos C, Nickles K, et al. In vitro efficacy of three different nonsurgical implant surface decontamination methods in three different defect configurations[J]. Int J Oral Maxillofac Implants, 2021, 36(2):271-280.
doi: 10.11607/jomi.8864 |
| [19] |
John G, Sahm N, Becker J, et al. Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study[J]. Clin Oral Investig, 2015, 19(8):1807-1814.
doi: 10.1007/s00784-015-1406-7 |
| [20] | Heitz-Mayfield LJ, Trombelli L, Heitz F, et al. A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis[J]. J Clin Perio-dontol, 2002, 29(Suppl 3):92-102. |
| [1] | Yao ZHANG,Jinxin GUO,Shijia ZHAN,Enyu HONG,Hui YANG,Anna JIA,Yan CHANG,Yongli GUO,Xuan ZHANG. Role and mechanism of cysteine and glycine-rich protein 2 in the malignant progression of neuroblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 495-504. |
| [2] | Fei SUN,Jian LIU,Si-qi LI,Yi-ping WEI,Wen-jie HU,Cui WANG. Profiles and differences of submucosal microbial in peri-implantitis and health implants: A cross-sectional study [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 30-37. |
| [3] | Ling-wei MENG,Xue LI,Sheng-han GAO,Yue LI,Rui-tao CAO,Yi ZHANG,Shao-xia PAN. Comparison of three methods for establishing rat peri-implantitis model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 22-29. |
| [4] | Zhong ZHANG,Huan-xin MENG,Jie HAN,Li ZHANG,Dong SHI. Effect of vertical soft tissue thickness on clinical manifestation of peri-implant tissue in patients with periodontitis [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 332-338. |
| [5] | Dong SHI,Jie CAO,Shi-ai DAI,Huan-xin MENG. Short-term outcome of regenerative surgery treating peri-implantitis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 58-63. |
| [6] | ZHANG Hai-dong, ZHANG Li, SHI Dong, HAN Jie, YAN Xia, XIE Ye-si, MENG Huan-xin. Clinical study of locking-taper implants in patients treated for periodontitis [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 300-307. |
|
||