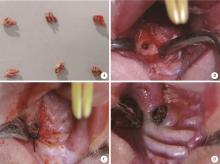
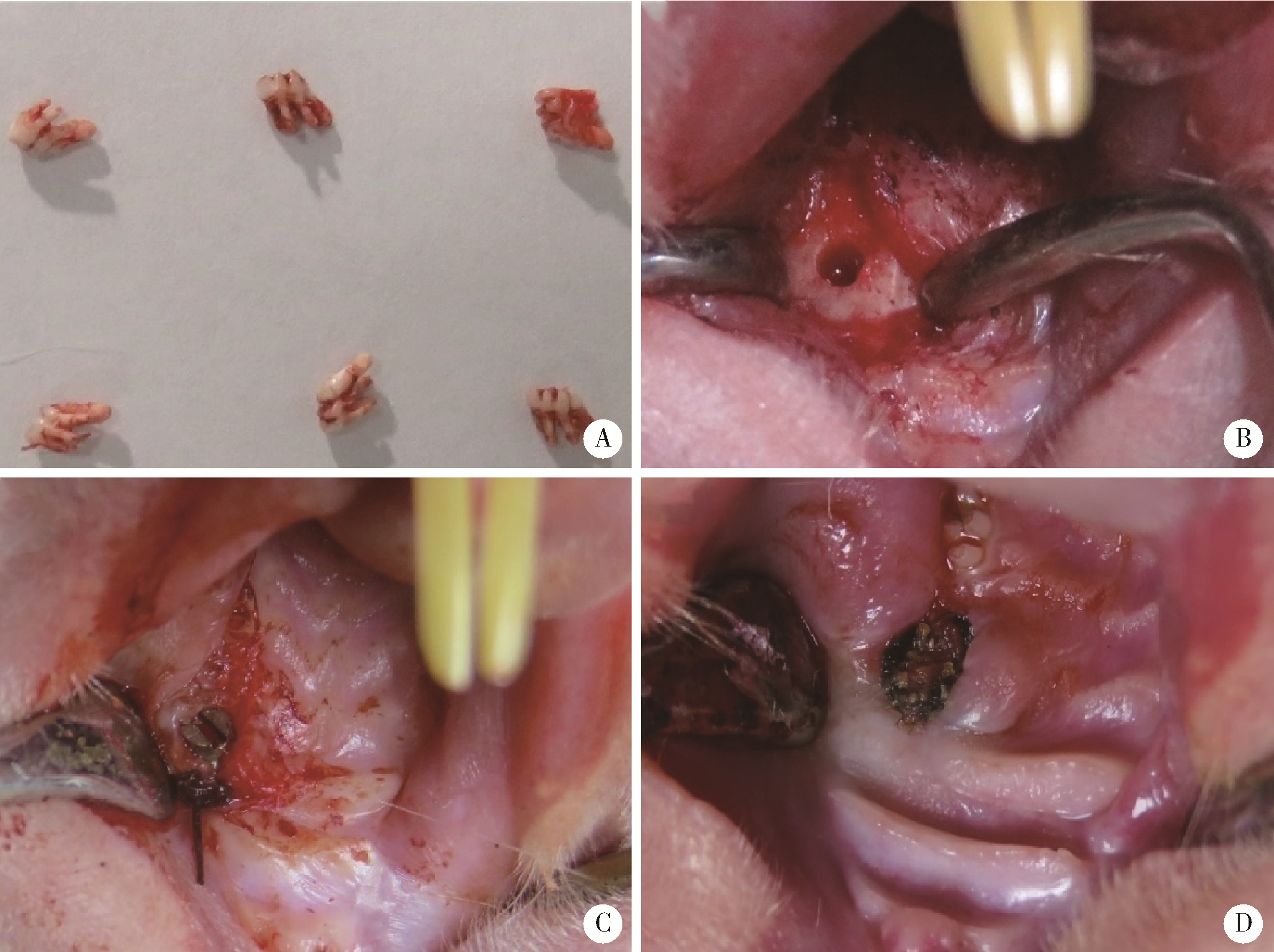
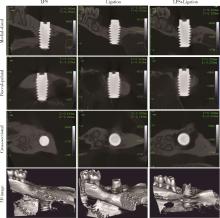
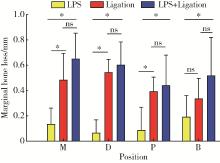
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (1): 22-29. doi: 10.19723/j.issn.1671-167X.2023.01.004
Previous Articles Next Articles
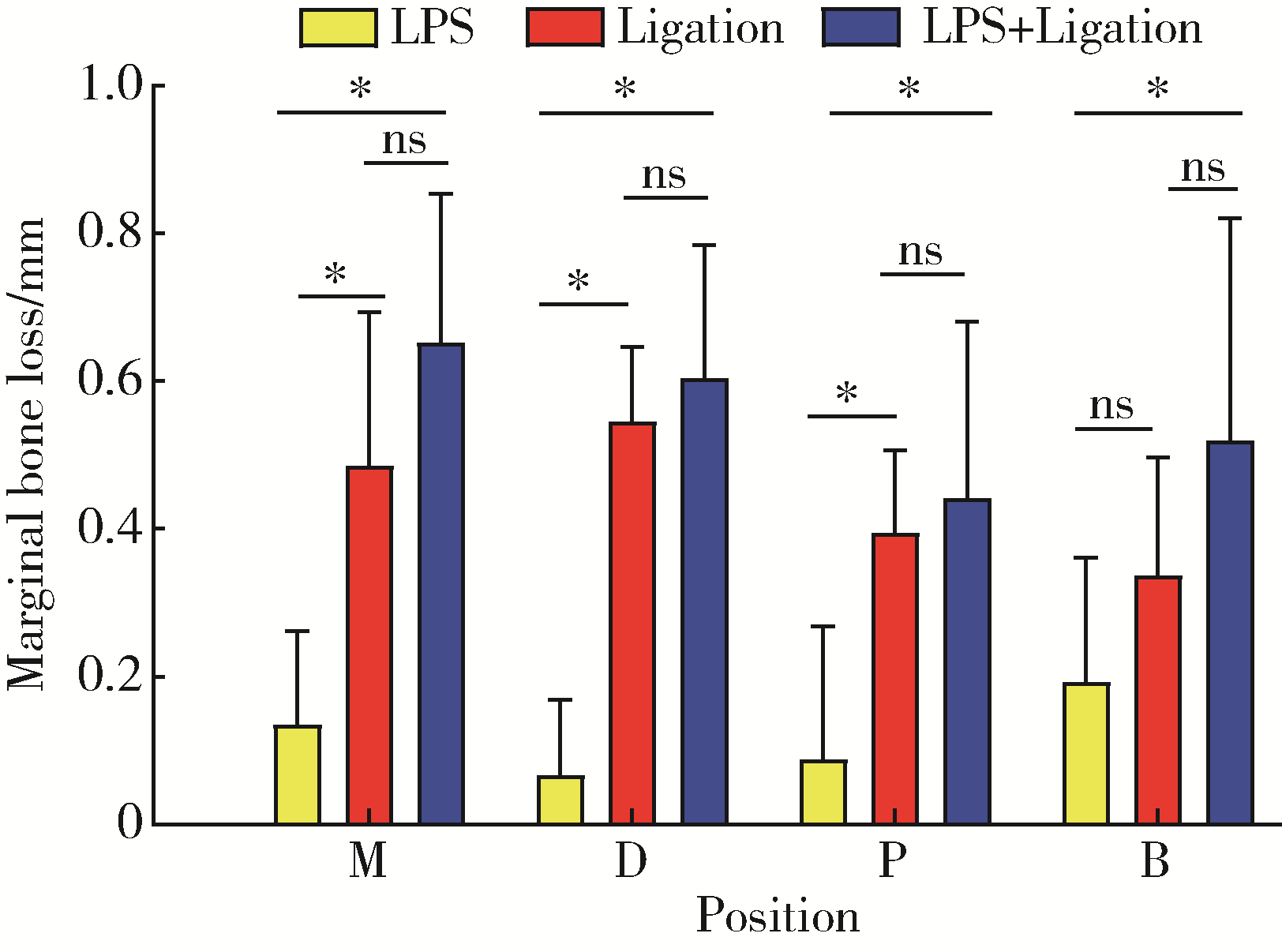
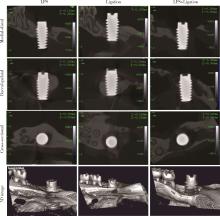
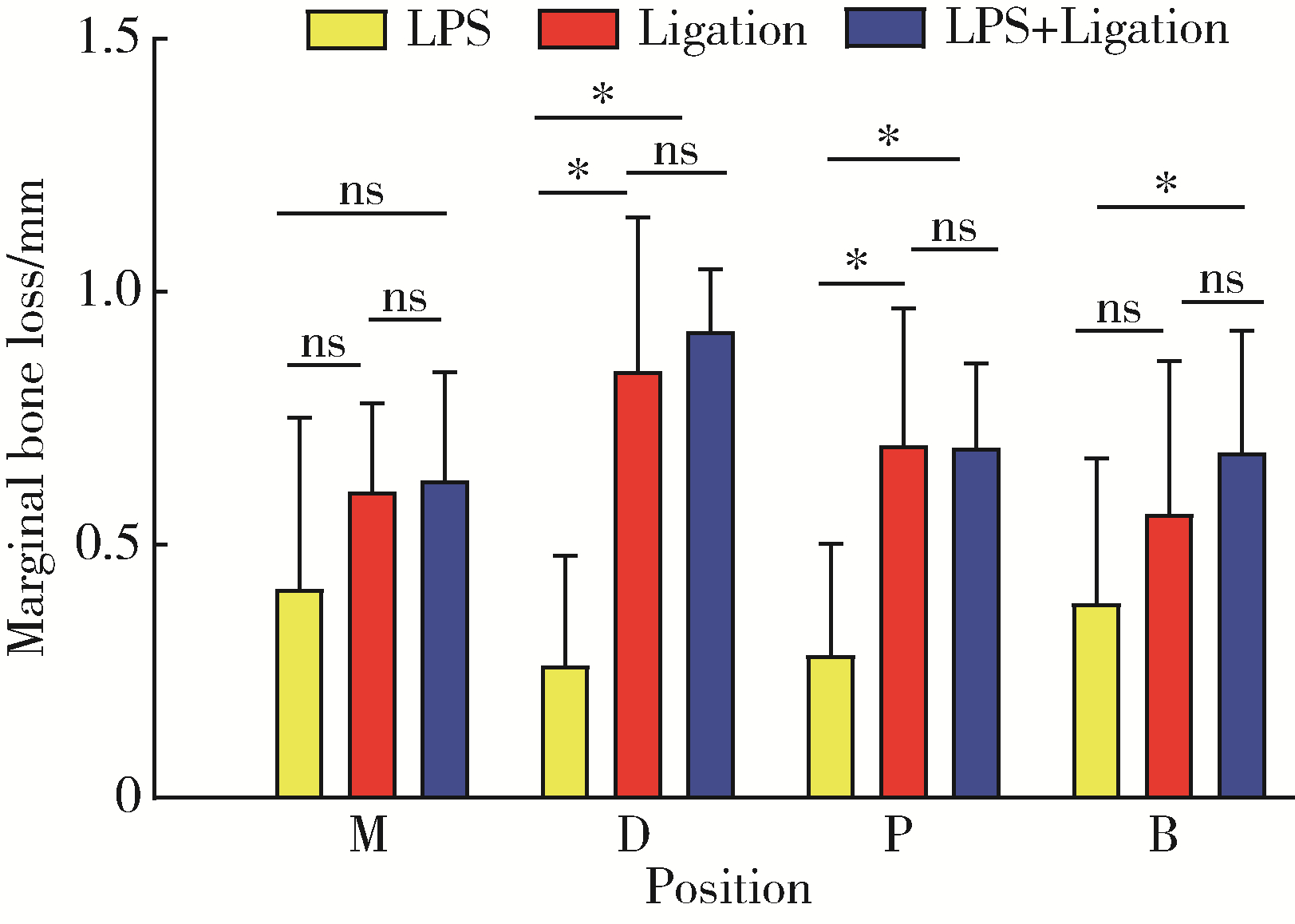
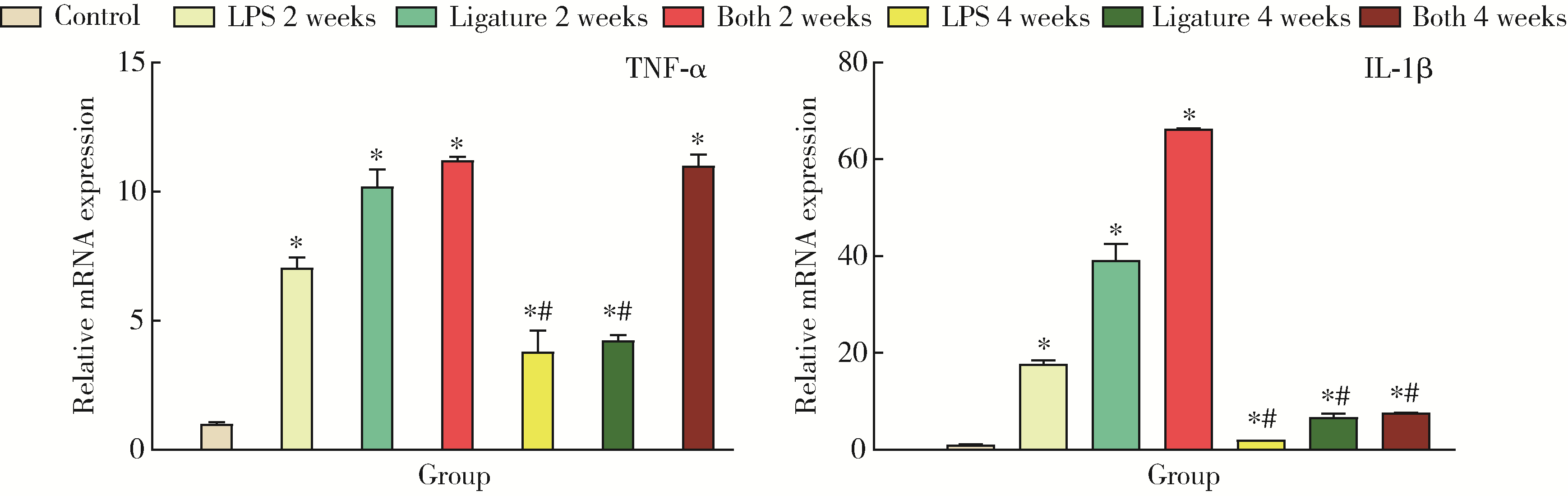
Comparison of three methods for establishing rat peri-implantitis model
Ling-wei MENG1,Xue LI2,Sheng-han GAO1,Yue LI1,Rui-tao CAO1,Yi ZHANG2,*( ),Shao-xia PAN1,*(
),Shao-xia PAN1,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digi-tal Medical Devices & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
2. Institute of Radiation Medicine, Academy of Military Medical Sciences, Beijing 100850, China
CLC Number:
- R781.42
| 1 | Schwarz F , Alcoforado G , Guerrero A , et al. Peri-implantitis: Summary and consensus statements of group 3. The 6th EAO Consensus Conference 2021[J]. Clin Oral Implants Res, 2021, 32 (Suppl 21): 245- 253. |
| 2 | Schliephake H , Sicilia A , Nawas BA , et al. Drugs and diseases: Summary and consensus statements of group 1. The 5 th EAO Consensus Conference 2018[J]. Clin Oral Implants Res, 2018, 29 (Suppl 18): 93- 99. |
| 3 | Roccuzzo M , Layton DM , Roccuzzo A , et al. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review[J]. Clin Oral Implants Res, 2018, 29 (Suppl 16): 331- 350. |
| 4 |
Fu JH , Wang HL . Breaking the wave of peri-implantitis[J]. Periodontol 2000, 2020, 84 (1): 145- 160.
doi: 10.1111/prd.12335 |
| 5 |
Wancket LM . Animal models for evaluation of bone implants and devices: Comparative bone structure and common model uses[J]. Vet Pathol, 2015, 52 (5): 842- 850.
doi: 10.1177/0300985815593124 |
| 6 |
Schwarz F , Sculean A , Engebretson SP , et al. Animal models for peri-implant mucositis and peri-implantitis[J]. Periodontol 2000, 2015, 68 (1): 168- 181.
doi: 10.1111/prd.12064 |
| 7 |
Kantarci A , Hasturk H , Van Dyke TE . Animal models for periodontal regeneration and peri-implant responses[J]. Periodontol 2000, 2015, 68 (1): 66- 82.
doi: 10.1111/prd.12052 |
| 8 | 朱白雪, 高晓蔚, 戴晓玮. 种植体周围炎动物模型的研究进展[J]. 口腔医学研究, 2018, 38 (8): 747- 751. |
| 9 |
Kensara A , Hefni E , Williams MA , et al. Microbiological profile and human immune response associated with peri-implantitis: A systematic review[J]. J Prosthodont, 2021, 30 (3): 210- 234.
doi: 10.1111/jopr.13270 |
| 10 |
Sun J , Eberhard J , Glage S , et al. Development of a peri-implantitis model in the rat[J]. Clin Oral Implants Res, 2020, 31 (3): 203- 214.
doi: 10.1111/clr.13556 |
| 11 | 杨少强, 廖旭辉, 顾为望, 等. 牙周炎犬种植体周围炎动物模型建立[J]. 口腔医学研究, 2011, 31 (12): 746- 750. |
| 12 | Miyamoto Y , Koretake K , Hirata M , et al. Influence of static overload on the bony interface around implants in dogs[J]. Int J Prosthodont, 2008, 21 (5): 437- 444. |
| 13 | 李星佳, 陈琪欣, 袁长永, 等. 种植体周围炎大鼠模型研究[J]. 口腔医学研究, 2021, 37 (4): 314- 318. |
| 14 |
Reinedahl D , Chrcanovic B , Albrektsson T , et al. Ligature-induced experimental peri-implantitis: A systematic review[J]. J Clin Med, 2018, 7 (12): 492.
doi: 10.3390/jcm7120492 |
| 15 |
Deng S , Hu Y , Zhou J , et al. TLR4 mediates alveolar bone resorption in experimental peri-implantitis through regulation of CD45(+) cell infiltration, RANKL/OPG ratio, and inflammatory cytokine production[J]. J Periodontol, 2020, 91 (5): 671- 682.
doi: 10.1002/JPER.18-0748 |
| 16 |
吴亚菲, 赵筱芩, 陈宇, 等. 不同方法建立大鼠实验性牙周炎模型的比较研究[J]. 四川大学学报(医学版), 2003, 34 (4): 742- 745.
doi: 10.3969/j.issn.1672-173X.2003.04.041 |
| 17 | He Q , Mu Z , Shrestha A , et al. Development of a rat model for type 2 diabetes mellitus peri-implantitis: A preliminary study[J]. Oral Dis, 2021, 28 (7): 1936- 1946. |
| 18 | Orecchioni M , Ghosheh Y , Pramod AB , et al. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages[J]. Front Immunol, 2019, 10, 1084. |
| 19 | 柯晓菁, 李厚轩, 闫福华, 等. 牙龈卟啉单胞菌脂多糖对骨髓来源的巨噬细胞和破骨细胞先天免疫反应的影响[J]. 中华口腔医学杂志, 2020, 55 (1): 32- 37. |
| 20 | Park SY , Kim KH , Rhee SH , et al. An immediate peri-implantitis induction model to study regenerative peri-implantitis treatments[J]. Clin Oral Implants Res, 2017, 28 (1): 36- 42. |
| 21 | Hasani-Sadrabadi MM , Sarrion P , Pouraghaei S , et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats[J]. Sci Transl Med, 2020, 12 (534): eaay6853. |
| [1] | Fei SUN,Jian LIU,Si-qi LI,Yi-ping WEI,Wen-jie HU,Cui WANG. Profiles and differences of submucosal microbial in peri-implantitis and health implants: A cross-sectional study [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 30-37. |
| [2] | HE Wei,YANG Si-wen,CHEN Juan,ZHU Xiao-jun,CHEN Zhi-zhong,MA Wen-jun. Effects of 275 nm and 310 nm ultraviolet irradiation on bone metabolism in ovariectomized osteoporotic rats [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 236-243. |
| [3] | Fei SUN,Si-qi LI,Yi-ping WEI,Jin-sheng ZHONG,Cui WANG,Wen-jie HU. Efficacy of combined application of glycine powder air-polishing in non-surgical treatment of peri-implant diseases [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 119-125. |
| [4] | WANG Gui-hong,ZUO Ting,LI Ran,ZUO Zheng-cai. Effect of rebamipide on the acute gouty arthritis in rats induced by monosodium urate crystals [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 716-720. |
| [5] | YIN Xue-qian, ZHANG Xiao-xuan, WEN Jing, LIU Si-qi, LIU Xin-ran, ZHOU Ruo-yu, WANG Jun-bo. Effects of the composite of buckwheat-oat-pea on blood glucose in diabetic rats [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 447-452. |
| [6] | Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA. Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 821-827. |
| [7] | Zhang-jian CHEN,Shuo HAN,Pai ZHENG,Shu-pei ZHOU,Guang JIA. Effect of subchronic combined oral exposure of titanium dioxide nanoparticles and glucose on levels of serum folate and vitamin B12 in young SD rats [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 451-456. |
| [8] | Shuo HAN,Zhang-jian CHEN,Di ZHOU,Pai ZHENG,Jia-he ZHANG,Guang JIA. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 457-463. |
| [9] | Zhong ZHANG,Huan-xin MENG,Jie HAN,Li ZHANG,Dong SHI. Effect of vertical soft tissue thickness on clinical manifestation of peri-implant tissue in patients with periodontitis [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 332-338. |
| [10] | Dong SHI,Jie CAO,Shi-ai DAI,Huan-xin MENG. Short-term outcome of regenerative surgery treating peri-implantitis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 58-63. |
| [11] | Shan-shan BAI,Si-yi MO,Xiao-xiang XU,Yun LIU,Qiu-fei XIE,Ye CAO. Characteristics of orofacial operant test for orofacial pain sensitivity caused by occlusal interference in rats [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 51-57. |
| [12] | Jiao HE,Ge-heng YUAN,Jun-qing ZHANG,Xiao-hui GUO. Approach to creating early diabetic peripheral neuropathy rat model [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1150-1154. |
| [13] | Wei WANG,Jin HOU,Wen-qiang HUANG. Temporary acceleration of interstitial fluid drainage in excited brain region induced by movement [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 206-209. |
| [14] | ZHANG Hai-dong, ZHANG Li, SHI Dong, HAN Jie, YAN Xia, XIE Ye-si, MENG Huan-xin. Clinical study of locking-taper implants in patients treated for periodontitis [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 300-307. |
| [15] | WANG Yu-jie, GUO Xiang-yang, WANG Jun. Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 310-314. |