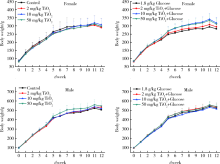
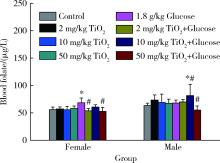
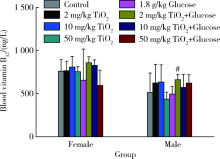
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 451-456. doi: 10.19723/j.issn.1671-167X.2020.03.009
Previous Articles Next Articles
Effect of subchronic combined oral exposure of titanium dioxide nanoparticles and glucose on levels of serum folate and vitamin B12 in young SD rats
Zhang-jian CHEN1,Shuo HAN1,Pai ZHENG1,Shu-pei ZHOU2,Guang JIA1,△( )
)
- 1. Department of Occupational and Enviromental Health Sciences, Peking University School of Public Health, Beijing 100191, China
2. Department of Laboratory Animal Science, Peking University Health Science Center, Beijing 100191, China
CLC Number:
- R994.4
| [1] | Lim JH, Bae D, Fong A. Titanium dioxide in food products: quantitative analysis using ICP-MS and Raman spectroscopy[J]. J Agric Food Chem, 2018,66(51):13533-13540. |
| [2] | FDA. Listing of color additives exempt from certification. In Code of Federal Regulations Title 21-Food and Drugs. 21 CFR 73.575. Washington, DC: US Government Printing Office, 2002. |
| [3] | Singh T, Shukla S, Kumar P, et al. Application of nanotechnology in food science: Perception and overview[J]. Front Microbiol, 2017(8):1501. |
| [4] | Yang Y, Doudrick K, Bi XY, et al. Characterization of food-grade titanium dioxide: The presence of nanosized particles[J]. Environ Sci Technol, 2014,48(11):6391-6400. |
| [5] |
Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products[J]. Environ Sci Technol, 2012,46(4):2242-2250.
pmid: 22260395 |
| [6] |
Winkler HC, Notter T, Meyer U, et al. Critical review of the safety assessment of titanium dioxide additives in food[J]. J Nanobiotechnology, 2018,16(1):51.
pmid: 29859103 |
| [7] |
Ruiz PA, Moron B, Becker HM, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome[J]. Gut, 2017,66(7):1216-1224.
pmid: 26848183 |
| [8] | Shrivastava R, Raza S, Yadav A, et al. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain[J]. Drug Chem Toxicol, 2014,37(3):336-347. |
| [9] |
Rollerova E, Tulinska J, Liskova A, et al. Titanium dioxide nanoparticles: Some aspects of toxicity/focus on the development[J]. Endocr Regul, 2015,49(2):97-112.
doi: 10.4149/endo_2015_02_97 pmid: 25960011 |
| [10] | Ervin RB, Kit BK, Carroll MD, et al. Consumption of added sugar among U.S. children and adolescents, 2005-2008[J]. NCHS data brief, 2012(87):1-8. |
| [11] |
Chen Z, Zhou D, Wang Y, et al. Combined effect of titanium dioxide nanoparticles and glucose on the cardiovascular system in young rats after oral administration[J]. J Appl Toxicol, 2019,39(4):590-602.
doi: 10.1002/jat.3750 pmid: 30427543 |
| [12] | Czajka M, Sawicki K, Sikorska K, et al. Toxicity of titanium dioxide nanoparticles in central nervous system[J]. Toxicol In Vitro, 2015,29(5):1042-1052. |
| [13] | Fan J, Ye J, Kamphorst JJ, et al. Quantitative flux analysis reveals folate-dependent NADPH production[J]. Nature, 2014,510(7504):298-302. |
| [14] | Chen Z, Wang Y, Zhuo L, et al. Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration[J]. Nanomedicine, 2015,11(7):1633-1642. |
| [15] |
Wang Y, Chen ZJ, Ba T, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles[J]. Small, 2013,9(9-10):1742-1752.
pmid: 22945798 |
| [16] |
Chen Z, Zhou D, Zhou S, et al. Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats[J]. J Appl Toxicol, 2019,39(5):807-819.
doi: 10.1002/jat.3769 pmid: 30644115 |
| [17] |
Chen ZJ, Wang Y, Wang X, et al. Effect of titanium dioxide nanoparticles on glucose homeostasis after oral administration[J]. J Appl Toxicol, 2018,38(6):810-823.
pmid: 29350773 |
| [1] | Hua ZHONG, Yuan LI, Liling XU, Mingxin BAI, Yin SU. Application of 18F-FDG PET/CT in rheumatic diseases [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 853-859. |
| [2] | Shengqi ZHENG,Tianchi HUA,Guicao YIN,Wei ZHANG,Ye YAO,Yifan LI. Association between the triglyceride-glucose index and the incidence of nephrolithiasis in male individuals [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 610-616. |
| [3] | Huangda GUO,Hexiang PENG,Siyue WANG,Tianjiao HOU,Yixin LI,Hanyu ZHANG,Mengying WANG,Yiqun WU,Xueying QIN,Xun TANG,Jing LI,Dafang CHEN,Yonghua HU,Tao WU. A ssociations of short-term ambient particulate matter exposure and MTNR1B gene with triglyceride-glucose index: A family-based study [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 375-383. |
| [4] | Zhan-yi ZHANG,Fan ZHANG,Ye YAN,Cai-guang CAO,Chang-jian LI,Shao-hui DENG,Yue-hao SUN,Tian-liang HUANG,Yun-he GUAN,Nan LI,Min LU,Zhen-hua HU,Shu-dong ZHANG. Near-infrared targeted probe designed for intraoperative imaging of prostatic neurovascular bundles [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 843-850. |
| [5] | Jia-qi SHI,Ying MA,Yi ZHANG,Zhang-jian CHEN,Guang JIA. Effects of titanium dioxide nanoparticles on circRNA expression profiles in human hepatocellular carcinoma cells HepG2 [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 392-399. |
| [6] | Yin-xiao BAI,Chun-yi LIU,Jie ZHANG,Wen-ying MENG,Lei JIN,Lei JIN. Association between periconceptional supplementation of folic acid or multiple-micronutrients containing folic acid and preterm delivery in women [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 495-501. |
| [7] | Ling-wei MENG,Xue LI,Sheng-han GAO,Yue LI,Rui-tao CAO,Yi ZHANG,Shao-xia PAN. Comparison of three methods for establishing rat peri-implantitis model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 22-29. |
| [8] | Yun-fei XING,Chun-yi LIU,Wen-ying MENG,Jie ZHANG,Ming-yuan JIAO,Lei JIN,Lei JIN. Relationship between micronutrients supplementation during periconceptional period and serum concentration of vitamin E in the 1st trimester of gestational period [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 434-442. |
| [9] | HE Wei,YANG Si-wen,CHEN Juan,ZHU Xiao-jun,CHEN Zhi-zhong,MA Wen-jun. Effects of 275 nm and 310 nm ultraviolet irradiation on bone metabolism in ovariectomized osteoporotic rats [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 236-243. |
| [10] | WANG Gui-hong,ZUO Ting,LI Ran,ZUO Zheng-cai. Effect of rebamipide on the acute gouty arthritis in rats induced by monosodium urate crystals [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 716-720. |
| [11] | YIN Xue-qian, ZHANG Xiao-xuan, WEN Jing, LIU Si-qi, LIU Xin-ran, ZHOU Ruo-yu, WANG Jun-bo. Effects of the composite of buckwheat-oat-pea on blood glucose in diabetic rats [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 447-452. |
| [12] | Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA. Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 821-827. |
| [13] | Lei JIN,Cheng WANG,Jie ZHANG,Wen-ying MENG,Jia-yu ZHANG,Jin-hui YU,Gui-yin LIN,Ming-kun TONG,Lei JIN. Maternal periconceptional folic acid supplementation and its effects on the prevalence of fetal neural tube defects [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 719-725. |
| [14] | Zhao-nian WANG,Wen-jing GAO,Bi-qi WANG,Wei-hua CAO,Jun LV,Can-qing YU,Zeng-chang PANG,Li-ming CONG,Hua WANG,Xian-ping WU,Yu LIU,Li-ming LI. Correlation between fasting plasma glucose, HbA1c and DNA methylation in adult twins [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 425-431. |
| [15] | Shuo HAN,Zhang-jian CHEN,Di ZHOU,Pai ZHENG,Jia-he ZHANG,Guang JIA. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 457-463. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 216
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 912
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||