Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 457-463. doi: 10.19723/j.issn.1671-167X.2020.03.010
Previous Articles Next Articles
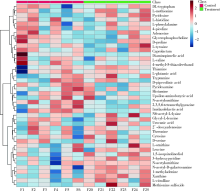
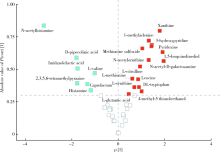
Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days
Shuo HAN,Zhang-jian CHEN,Di ZHOU,Pai ZHENG,Jia-he ZHANG,Guang JIA( )
)
- Department of Occupational and Environmental Health Sciences, Peking University School of Public Health, Beijing 100191, China
CLC Number:
- R994.4
| [1] | Ropers MH, Terrisse H, Mercier-Bonin M, et al. Titanium dio-xide as food additive[M]. Magdalena Janus: InTech Rijeka, 2017. |
| [2] |
Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, et al. Effects of titanium dioxide nanoparticles exposure on human health: A review[J]. Biol Trace Elem Res, 2020,193(1):118-129.
doi: 10.1007/s12011-019-01706-6 pmid: 30982201 |
| [3] |
Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products[J]. Environ Sci Technol, 2012,46(4):2242-2250.
pmid: 22260395 |
| [4] | Yang Y, Doudrick K, Bi X, et al. Characterization of food-grade titanium dioxide: the presence of nanosized particles[J]. Environ Sci Technol, 2014,48(11):6391-6400. |
| [5] |
Winkler HC, Notter T, Meyer U, et al. Critical review of the safety assessment of titanium dioxide additives in food[J]. J Nanobiotechnology, 2018,16(1):51.
pmid: 29859103 |
| [6] |
Wang J, Zhou G, Chen C, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration[J]. Toxicol Lett, 2007,168(2):176-185.
pmid: 17197136 |
| [7] | Shukla RK, Kumar A, Gurbani D, et al. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells[J]. Nanotoxicology, 2013,7(1):48-60. |
| [8] |
Bachler G, von Goetz N, Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles[J]. Nanotoxicology, 2015,9(3):373-380.
pmid: 25058655 |
| [9] | Karu N, Deng L, Slae M, et al. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database[J]. Anal Chim Acta, 2018(1030):1-24. |
| [10] |
Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms[J]. Nat Rev Mol Cell Biol, 2016,17(7):451-459.
doi: 10.1038/nrm.2016.25 pmid: 26979502 |
| [11] | 周迪, 陈章健, 胡贵平, 等. 纳米二氧化钛亚急性经口暴露对大鼠氧化/抗氧化生物标志和炎性因子的影响[J/OL]. 北京大学学报(医学版), ( 2018- 11- 09) [2019-12-28]. http://kns.cnki.net/kcms/detail/11.4691.R.20181108.1357.010.html. |
| [12] | Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature, 2006,444(7122):1027-1031. |
| [13] | Brun E, Barreau F, Veronesi G, et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia[J]. Part Fibre Toxicol, 2014(11):13. |
| [14] | Guo Z, Martucci NJ, Moreno-Olivas F, et al. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine[J]. NanoImpact, 2017(5):70-82. |
| [15] | Pinget G, Tan J, Janac B, et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction[J]. Front Nutr, 2019(6):57. |
| [16] | Di Z, Shuo H, Tenglong Y, et al. Toxicity of titanium dioxide nanoparticles induced by reactive oxygen species[J]. Reactive Oxygen Species, 2019,8(23):267-275. |
| [17] | Chen Z, Wang Y, Wang X, et al. Effect of titanium dioxide nanoparticles on glucose homeostasis after oral administration[J]. J Appl Toxicol, 2018,38(6):810-823. |
| [18] | Bull MJ, Plummer NT. Part 1: The human gut microbiome in health and disease[J]. Integr Med (Encinitas), 2014,13(6):17-22. |
| [19] | Weissbach H, Redfield BG, Axelrod J. The exzymic acetylation of serotonin and other naturally occurring amines[J]. Biochim Biophys Acta, 1961(54):190-192. |
| [20] | Xiao F, Yu J, Guo Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice[J]. Metabolism, 2014,63(6):841-850. |
| [21] |
Taya Y, Ota Y, Wilkinson AC, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation[J]. Science, 2016,354(6316):1152-1155.
pmid: 27934766 |
| [22] |
Duan Y, Liu J, Ma L, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice[J]. Biomaterials, 2010,31(5):894-899.
pmid: 19857890 |
| [23] |
Lee BC, Dikiy A, Kim HY, et al. Functions and evolution of selenoprotein methionine sulfoxide reductases[J]. Biochim Biophys Acta, 2009,1790(11):1471-1477.
pmid: 19406207 |
| [24] | Zierer J, Jackson MA, Kastenmüller G, et al. The fecal metabolome as a functional readout of the gut microbiome[J]. Nat Genet, 2018,50(6):790-795. |
| [25] | Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health[J]. Front Microbiol, 2016(7):1144. |
| [26] | Xia Y, Dawson VL, Dawson TM, et al. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury[J]. Proc Natl Acad Sci U S A, 1996,93(13):6770-6774. |
| [27] | Tiwari S, van Tonder AJ, Vilchèze C, et al. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis[J]. Proc Natl Acad Sci USA, 2018,115(39):9779-9784. |
| [1] | Zhan-yi ZHANG,Fan ZHANG,Ye YAN,Cai-guang CAO,Chang-jian LI,Shao-hui DENG,Yue-hao SUN,Tian-liang HUANG,Yun-he GUAN,Nan LI,Min LU,Zhen-hua HU,Shu-dong ZHANG. Near-infrared targeted probe designed for intraoperative imaging of prostatic neurovascular bundles [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 843-850. |
| [2] | Jia-qi SHI,Ying MA,Yi ZHANG,Zhang-jian CHEN,Guang JIA. Effects of titanium dioxide nanoparticles on circRNA expression profiles in human hepatocellular carcinoma cells HepG2 [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 392-399. |
| [3] | Ling-wei MENG,Xue LI,Sheng-han GAO,Yue LI,Rui-tao CAO,Yi ZHANG,Shao-xia PAN. Comparison of three methods for establishing rat peri-implantitis model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 22-29. |
| [4] | Xue-ping WANG,Yu-ya-nan ZHANG,Tian-lan LU,Zhe LU,Zhe-wei KANG,Yao-yao SUN,Wei-hua YUE. Variations in fecal microbiota of first episode schizophrenia associated with clinical assessment and serum metabolomics [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 863-873. |
| [5] | Yuan MA,Yue ZHANG,Rui LI,Shu-wei DENG,Qiu-shi QIN,Liu-luan ZHU. Characteristics of amino acid metabolism in myeloid-derived suppressor cells in septic mice [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 532-540. |
| [6] | HE Wei,YANG Si-wen,CHEN Juan,ZHU Xiao-jun,CHEN Zhi-zhong,MA Wen-jun. Effects of 275 nm and 310 nm ultraviolet irradiation on bone metabolism in ovariectomized osteoporotic rats [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 236-243. |
| [7] | WANG Gui-hong,ZUO Ting,LI Ran,ZUO Zheng-cai. Effect of rebamipide on the acute gouty arthritis in rats induced by monosodium urate crystals [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 716-720. |
| [8] | YIN Xue-qian, ZHANG Xiao-xuan, WEN Jing, LIU Si-qi, LIU Xin-ran, ZHOU Ruo-yu, WANG Jun-bo. Effects of the composite of buckwheat-oat-pea on blood glucose in diabetic rats [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 447-452. |
| [9] | Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA. Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 821-827. |
| [10] | Zhang-jian CHEN,Shuo HAN,Pai ZHENG,Shu-pei ZHOU,Guang JIA. Effect of subchronic combined oral exposure of titanium dioxide nanoparticles and glucose on levels of serum folate and vitamin B12 in young SD rats [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 451-456. |
| [11] | Shan-shan BAI,Si-yi MO,Xiao-xiang XU,Yun LIU,Qiu-fei XIE,Ye CAO. Characteristics of orofacial operant test for orofacial pain sensitivity caused by occlusal interference in rats [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 51-57. |
| [12] | Jiao HE,Ge-heng YUAN,Jun-qing ZHANG,Xiao-hui GUO. Approach to creating early diabetic peripheral neuropathy rat model [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1150-1154. |
| [13] | Wei WANG,Jin HOU,Wen-qiang HUANG. Temporary acceleration of interstitial fluid drainage in excited brain region induced by movement [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 206-209. |
| [14] | WANG Yu-jie, GUO Xiang-yang, WANG Jun. Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 310-314. |
| [15] | ZHANG Jie, SONG Lei, DUAN Deng-hui, YUE Lin. Observation of oral Streptococcus oligofermentans colonization in rats [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 316-319. |
|
||