Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (4): 716-720. doi: 10.19723/j.issn.1671-167X.2021.04.016
Previous Articles Next Articles
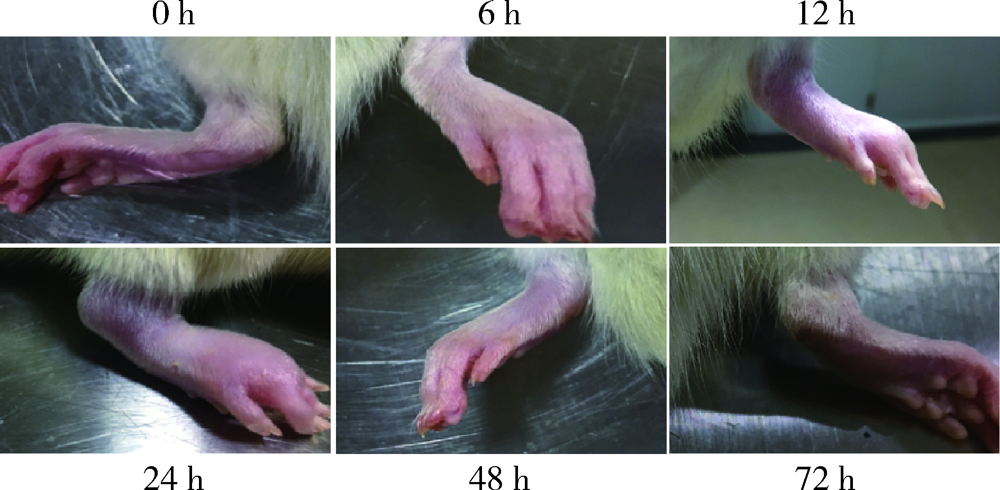
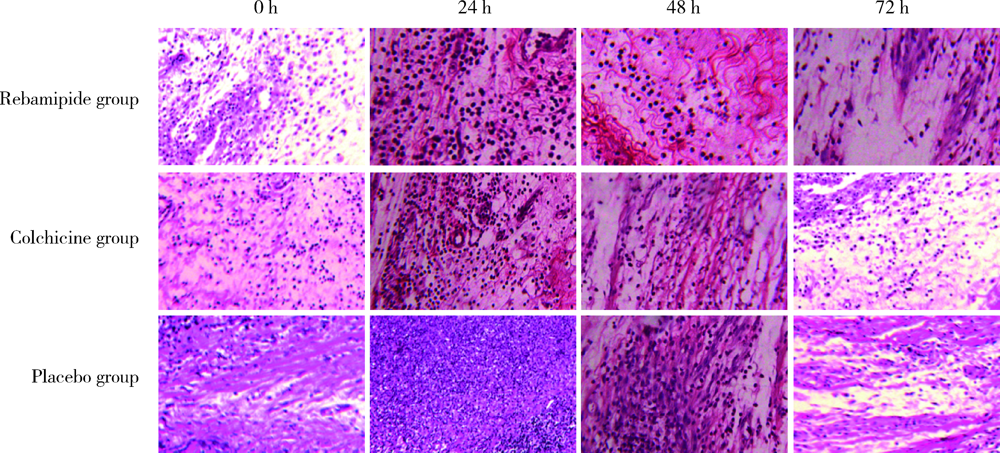
Effect of rebamipide on the acute gouty arthritis in rats induced by monosodium urate crystals
WANG Gui-hong( ),ZUO Ting,LI Ran,ZUO Zheng-cai
),ZUO Ting,LI Ran,ZUO Zheng-cai
- Department of Rheumatology and Immunology, Anqing Hospital of Anhui Medical University, Anqing 246000, Anhui, China
CLC Number:
- R589.7
| [1] | 路杰, 崔凌凌, 李长贵, 等. 原发性痛风流行病学研究进展 [J]. 中华内科杂志, 2015, 54(3):244-247. |
| [2] | 中华医学会风湿病学分会. 2016中国痛风诊疗指南 [J]. 中华内科杂志, 2016, 55(11):892-899. |
| [3] |
Kim SK, Choe JY, Park KY. Rebamipide suppresses monosodium urate crystal-induced interleukin-1β production through regulation of oxidative stress and Caspase-1 in THP-1 cells [J]. Inflammation, 2016, 39(1):473-482.
doi: 10.1007/s10753-015-0271-5 |
| [4] |
Moon SJ, Woo YJ, Jeong JH, et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes [J]. Osteoarthritis Cartilage, 2012, 20(11):1426-1438.
doi: 10.1016/j.joca.2012.08.002 |
| [5] |
Kohashi M, Ishimaru N, Arakaki R, et al. Effective treatment with oral administration of rebamipide in a mouse model of Sjögren’s syndrome [J]. Arthritis Rheum, 2008, 58(2):389-400.
doi: 10.1002/(ISSN)1529-0131 |
| [6] |
Coderre TJ, Wall PD. Ankle joint urate arthritis (AJUA) in rats: an alternative animal model of arthritis to that produced by Freund’s adjuvant [J]. Pain, 1987, 28(3):379-393.
pmid: 3574965 |
| [7] |
Park JS, Oh Y, Park O, et al. PEGylated TRAIL ameliorates experimental inflammatory arthritis by regulation of Th17 cells and regulatory T cells [J]. J Control Release, 2017, 267:163-171.
doi: 10.1016/j.jconrel.2017.10.004 |
| [8] |
Hartmann P, Szabó A, Eros G, et al. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats [J]. Eur J Pharmacol, 2009, 622(1/3):58-64.
doi: 10.1016/j.ejphar.2009.09.012 |
| [9] |
Tramontini N, Huber C, Liu-Bryan R, et al. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis [J]. Arthritis Rheum, 2004, 50(8):2633-2639.
doi: 10.1002/(ISSN)1529-0131 |
| [10] |
Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation [J]. Immunol Rev, 2010, 233(1):218-232.
doi: 10.1111/j.0105-2896.2009.00860.x pmid: 20193002 |
| [11] | Zhang Y, Zheng Y. Effects and mechanisms of potent caspase 1 inhibitor VX765 treatment on collagen-induced arthritis in mice [J]. Clin Exp Rheumatolm, 2016, 34(1):111-118. |
| [12] |
Jhang JJ, Lu CC, Yen GC. Epigallocatechin gallate inhibits urate crystals-induced peritoneal inflammation in C57BL/6 mice [J]. Mol Nutr Food Res, 2016, 60(10):2297-2303.
doi: 10.1002/mnfr.v60.10 |
| [13] |
Zhang S, Qing Q, Bai Y, et al. Rebamipide helps defend against nonsteroidal anti-inflammatory drugs induced gastroenteropathy: A systematic review and meta-analysis [J]. Dig Dis Sci, 2013, 58(7):1991-2000.
doi: 10.1007/s10620-013-2606-0 |
| [14] |
Choe JY, Park KY, Lee SJ, et al. Rebamipide inhibits tumor necrosis factor-α-induced interleukin-8 expression by suppressing the NF-κB signal pathway in human umbilical vein endothelial cells [J]. Inflamm Res, 2010, 59(12):1019-1026.
doi: 10.1007/s00011-010-0221-5 |
| [15] |
Kim H, Seo JY, Kim KH. Inhibition of lipid peroxidation, NFkappaB activation and IL-8 production by rebamipide in Helicobacter pylori-stimulated gastric epithelial cells [J]. Dig Dis Sci, 2000, 45(3):621-628.
doi: 10.1023/A:1005474013988 |
| [16] |
Funahashi Y, Yoshida M, Yamamoto T, et al. Intravesical application of rebamipide suppresses bladder inflammation in a rat cystitis model [J]. J Urol, 2014, 191(4):1147-1152.
doi: 10.1016/j.juro.2013.11.026 pmid: 24262494 |
| [17] |
Suzuki Y, Hasegawa M, Matsui Y, et al. Intra-articular injection of rebamipide prevents articular cartilage degeneration in murine post-traumatic osteoarthritis models [J]. Mod Rheumatol, 2020, 30(4):765-772.
doi: 10.1080/14397595.2019.1641912 |
| [1] | Zhan-yi ZHANG,Fan ZHANG,Ye YAN,Cai-guang CAO,Chang-jian LI,Shao-hui DENG,Yue-hao SUN,Tian-liang HUANG,Yun-he GUAN,Nan LI,Min LU,Zhen-hua HU,Shu-dong ZHANG. Near-infrared targeted probe designed for intraoperative imaging of prostatic neurovascular bundles [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 843-850. |
| [2] | Ling-wei MENG,Xue LI,Sheng-han GAO,Yue LI,Rui-tao CAO,Yi ZHANG,Shao-xia PAN. Comparison of three methods for establishing rat peri-implantitis model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 22-29. |
| [3] | HE Wei,YANG Si-wen,CHEN Juan,ZHU Xiao-jun,CHEN Zhi-zhong,MA Wen-jun. Effects of 275 nm and 310 nm ultraviolet irradiation on bone metabolism in ovariectomized osteoporotic rats [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 236-243. |
| [4] | YIN Xue-qian, ZHANG Xiao-xuan, WEN Jing, LIU Si-qi, LIU Xin-ran, ZHOU Ruo-yu, WANG Jun-bo. Effects of the composite of buckwheat-oat-pea on blood glucose in diabetic rats [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 447-452. |
| [5] | Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA. Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 821-827. |
| [6] | Shuo HAN,Zhang-jian CHEN,Di ZHOU,Pai ZHENG,Jia-he ZHANG,Guang JIA. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 457-463. |
| [7] | Zhang-jian CHEN,Shuo HAN,Pai ZHENG,Shu-pei ZHOU,Guang JIA. Effect of subchronic combined oral exposure of titanium dioxide nanoparticles and glucose on levels of serum folate and vitamin B12 in young SD rats [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 451-456. |
| [8] | Shan-shan BAI,Si-yi MO,Xiao-xiang XU,Yun LIU,Qiu-fei XIE,Ye CAO. Characteristics of orofacial operant test for orofacial pain sensitivity caused by occlusal interference in rats [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 51-57. |
| [9] | Jiao HE,Ge-heng YUAN,Jun-qing ZHANG,Xiao-hui GUO. Approach to creating early diabetic peripheral neuropathy rat model [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1150-1154. |
| [10] | Wei WANG,Jin HOU,Wen-qiang HUANG. Temporary acceleration of interstitial fluid drainage in excited brain region induced by movement [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 206-209. |
| [11] | WANG Yu-jie, GUO Xiang-yang, WANG Jun. Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 310-314. |
| [12] | ZHANG Jie, SONG Lei, DUAN Deng-hui, YUE Lin. Observation of oral Streptococcus oligofermentans colonization in rats [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 316-319. |
| [13] | ZHENG Shao-Qiang, CHEN Xue, WANG Ya-Jie, AN Li-Xin. Effects of sevoflurane on brain neuroapoptosis and ability of long-term learning and memory in newborn rats [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 674-678. |
| [14] | YANG Jun, WANG Tao, ZHANG Yan, XU Hua, WANG Shao-Gang, LIU Ji-Hong, YE Zhang-Qun. Concentration of basic fibroblast growth factor in plasma and cavernous corpus of aged rat [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 582-585. |
| [15] | ZHANG Jin, XING Yan, WANG Xin-Li, GUAN Yu-Hong, ZHANG Hui. Decreased insulin sensitivity in rat hepatocytes with intrauterine growth retardation and establishment of insulin resistance cell model in vitro [J]. Journal of Peking University(Health Sciences), 2014, 46(3): 464-468. |
|
||