Journal of Peking University (Health Sciences) ›› 2026, Vol. 58 ›› Issue (1): 175-183. doi: 10.19723/j.issn.1671-167X.2026.01.023
Previous Articles Next Articles
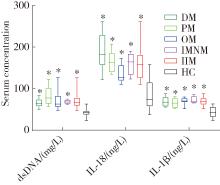
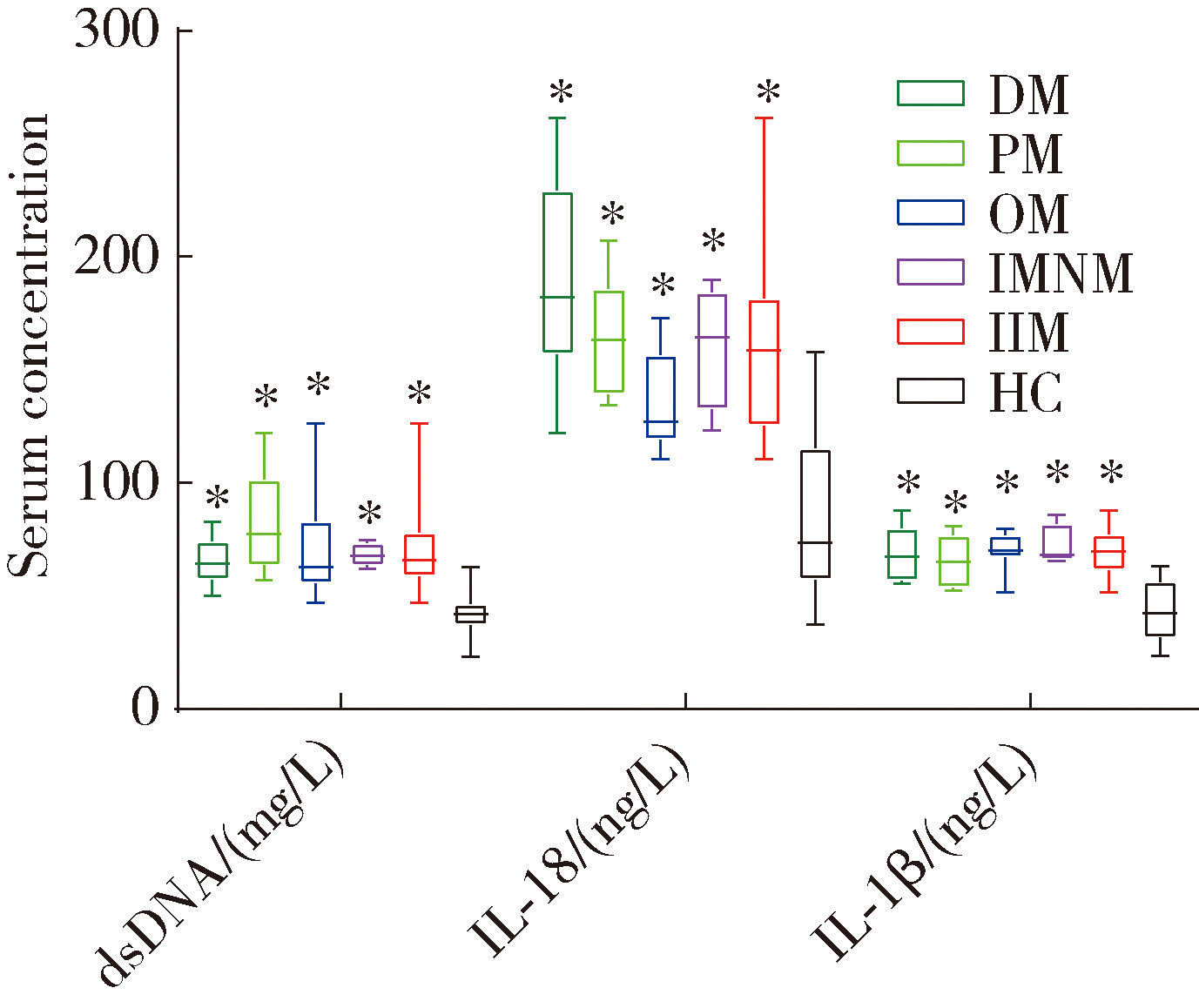
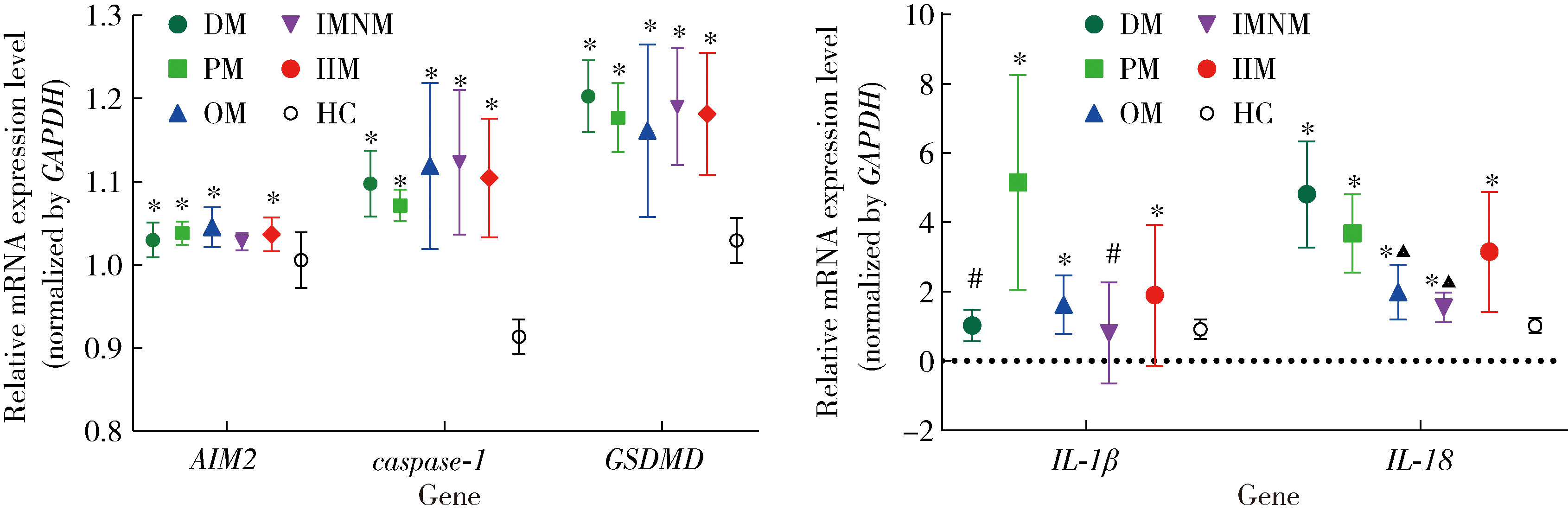
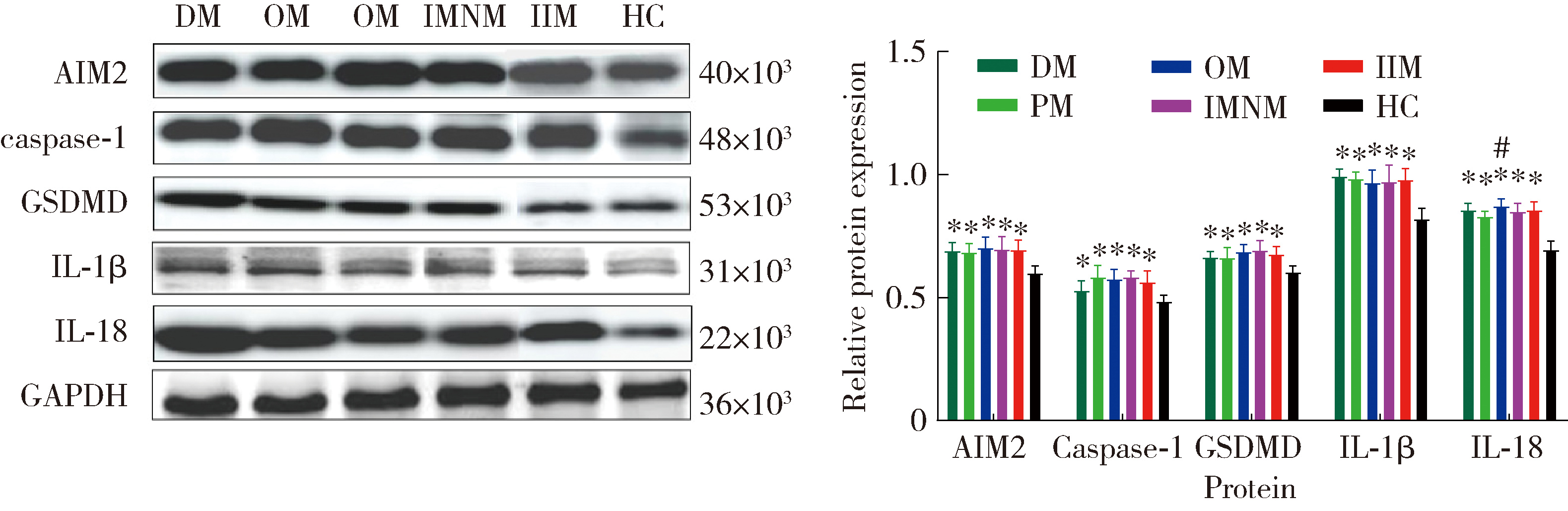
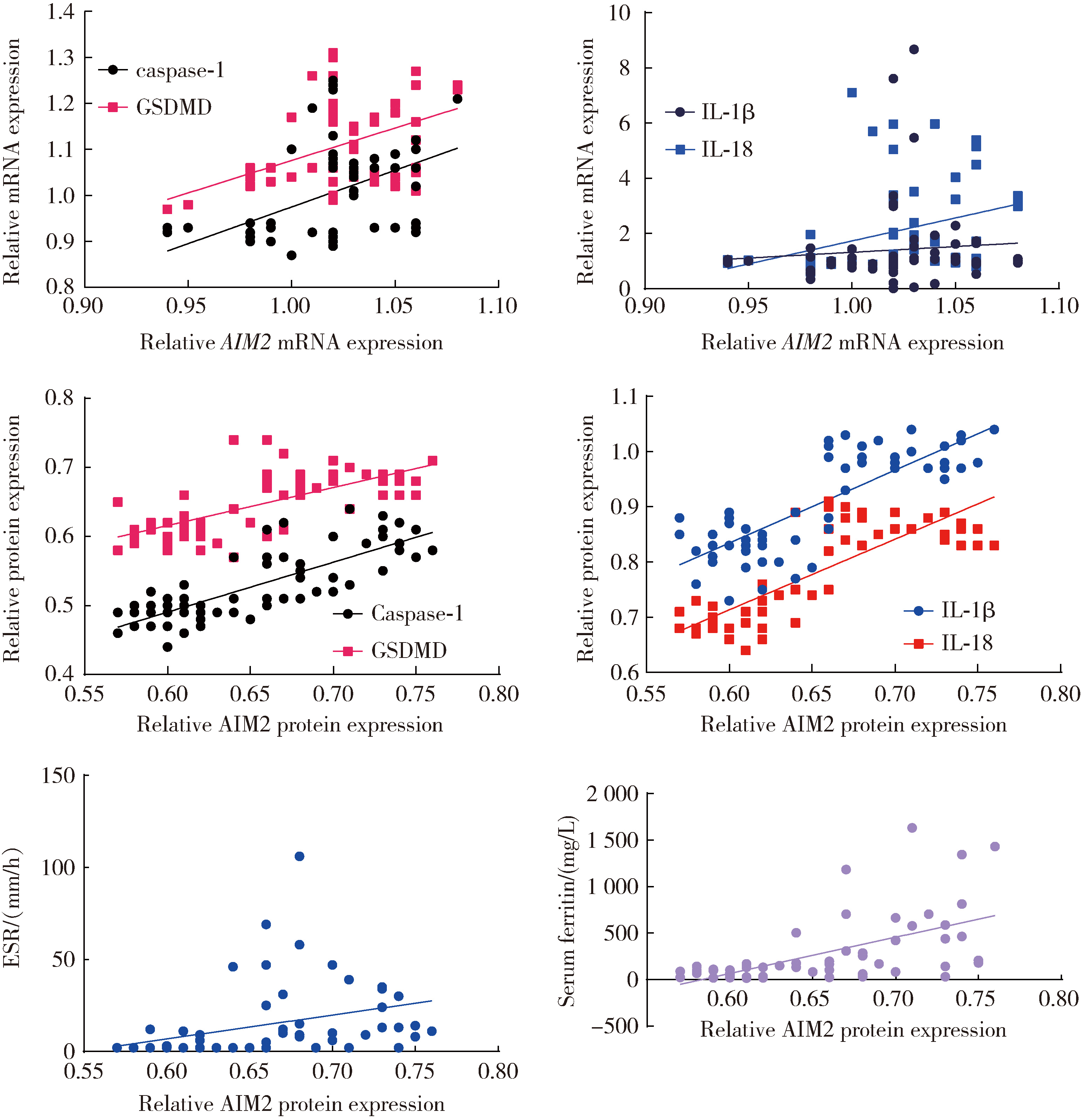
Expression of the melanoma 2-mediated pyroptosis pathway in peripheral blood mononuclear cells of patients with idiopathic inflammatory myopathies
Jiyan CHU1,2, Ping LI1,*( ), Jing TIAN3, Diyu FU1,2, Lin GUO1, Rui SUN1, Yadi LI1
), Jing TIAN3, Diyu FU1,2, Lin GUO1, Rui SUN1, Yadi LI1
- 1. Department of Rheumatology and Immunology, General Hospital of Northern Theater Command, Shenyang 110001, China
2. Graduate School, Dalian Medical University, Dalian 116044, Liaoning, China
3. Department of Orthopedics, General Hospital of Northern Theater Command, Shenyang 110001, China
CLC Number:
- R593.26
| 1 |
doi: 10.1136/annrheumdis-2013-205127 |
| 2 |
doi: 10.1111/j.1756-185X.2011.01669.x |
| 3 |
doi: 10.1016/S1474-4422(18)30254-0 |
| 4 |
doi: 10.1002/art.40320 |
| 5 |
doi: 10.1002/eji.201848070 |
| 6 |
doi: 10.1016/j.clim.2016.12.011 |
| 7 |
doi: 10.1016/j.imbio.2019.11.015 |
| 8 |
doi: 10.1016/j.jaut.2019.102381 |
| 9 |
doi: 10.1186/s13075-016-1033-y |
| 10 |
doi: 10.1016/j.nmd.2017.09.016 |
| 11 |
刘宁, 吴婵媛, 王迁, 等. 特发性炎性肌病核心评估指标[J]. 中华临床免疫和变态反应杂志, 2019, 13 (4): 318- 321.
|
| 12 |
潘蕾, 谢娟, 李嘉欣, 等. 特发性炎性肌病的评估与监测[J]. 临床内科杂志, 2023, 40 (3): 148- 151.
|
| 13 |
陈梦雅, 郑捷, 曹华. 皮肌炎皮损评分方法的临床应用[J]. 中华皮肤科杂志, 2017, 50 (1): 70- 72.
|
| 14 |
doi: 10.1111/bjd.18600 |
| 15 |
doi: 10.1093/rheumatology/keaa473 |
| 16 |
doi: 10.4103/0366-6999.180528 |
| 17 |
doi: 10.1172/jci.insight.134189 |
| 18 |
doi: 10.1002/art.41078 |
| 19 |
doi: 10.1016/j.immuni.2012.02.014 |
| 20 |
doi: 10.1038/nature07725 |
| 21 |
doi: 10.1189/jlb.3MR0516-224R |
| 22 |
doi: 10.1038/s41598-022-22754-4 |
| 23 |
doi: 10.1002/art.41639 |
| 24 |
doi: 10.1111/j.1365-2249.2006.03180.x |
| 25 |
doi: 10.1093/rheumatology/key222 |
| 26 |
doi: 10.1172/jci.insight.139558 |
| 27 |
|
| 28 |
doi: 10.1038/s41577-019-0228-2 |
| 29 |
doi: 10.1016/j.intimp.2021.107810 |
| 30 |
邓蕊, 柴克霞. 细胞焦亡非经典途径蛋白质在皮肌炎/多发性肌炎患者肌肉组织中的表达及意义[J]. 中华微生物学和免疫学杂志, 2021, 41 (10): 771- 777.
|
| [1] | Yirui LIAN, Jingxuan LIU, Liang ZHAO, Jing ZHAO, Sitian ZANG, Yuhui LI. Rheumatic disease spectrum and immunological profile of anti-PM/Scl antibodies in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1018-1023. |
| [2] | YI Wen-xia,WEI Cui-jie,WU Ye,BAO Xin-hua,XIONG Hui,CHANG Xing-zhi. Long-term rituximab treatment of refractory idiopathic inflammatory myopathy: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1191-1195. |
| [3] | XIAO Yun-shu,ZHU Feng-yun-zhi,LUO Lan,XING Xiao-yan,LI Yu-hui,ZHANG Xue-wu,SHEN Dan-hua. Clinical and immunological characteristics of 88 cases of overlap myositis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1088-1093. |
|
||