Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (5): 797-804. doi: 10.19723/j.issn.1671-167X.2019.05.002
Previous Articles Next Articles
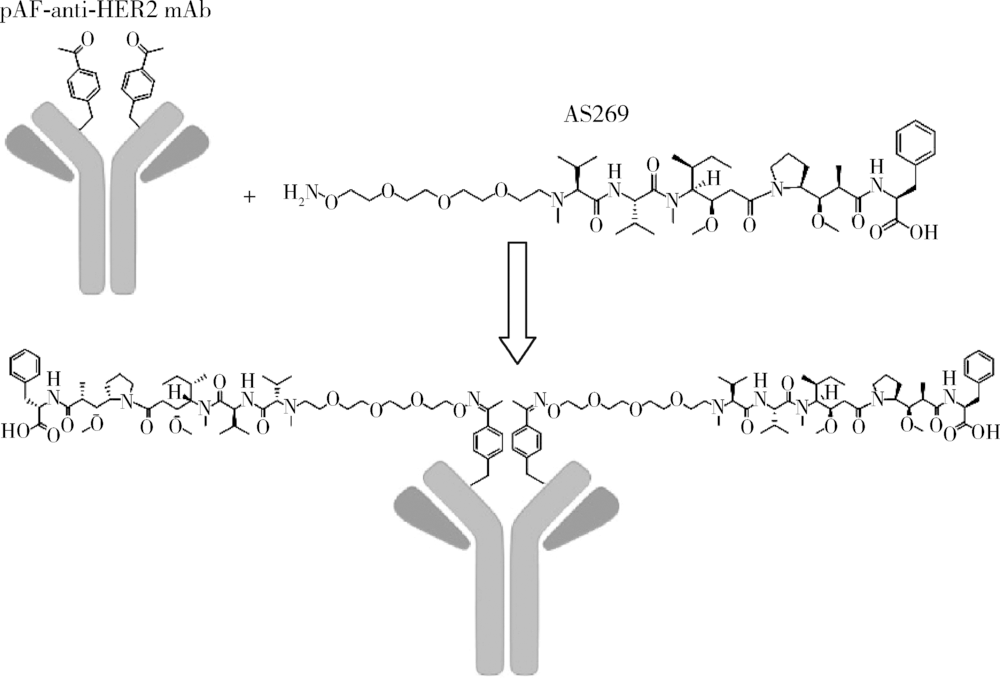
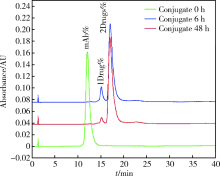
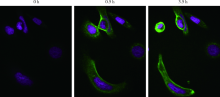
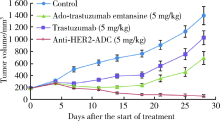
Pharmacological effects of site specific conjugated anti-human epidermal growth factor receptor 2-antibody drug conjugate using unnatural amino acid technology
Xue-jun LIANG1,Li-ying GONG1,Fei ZHOU1,De-min ZHOU2,Jing-jing ZHU1,△( )
)
- 1. Zhejiang NovoCodex Biopharmaceuticals Company Limited, Shaoxing, Zhejiang 312000, China
2. Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing 100871, China
CLC Number:
- R966
| [1] | Peters C, Brown S . Antibody-drug conjugates as novel anti-cancer chemotherapeutics[J]. Biosci Rep, 2015,35(4):1042-1061. |
| [2] | Bouchard H, Viskov C, Garcia-Echeverria C . Antibody-drug conjugates: a new wave of cancer drugs[J]. Bioorg Med Chemi Lett, 2014,24(23):5357-5363. |
| [3] | Hamblett KJ, Senter PD, Chace DF , et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate[J]. Clin Cancer Res, 2004,10(20):7063-7070. |
| [4] | Wang L, Amphlett G, Blättler WA , et al. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry[J]. Protein Sci, 2005,14(9):2436-2446. |
| [5] | Sun MM, Beam KS, Cerveny CG , et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides[J]. Bioconjug Chem, 2005,16(5):1282-1290. |
| [6] | Junutula JR, Raab H, Clark S , et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index[J]. Nat Biotechnol, 2008,26(8):925-932. |
| [7] | Tian F, Lu Y, Manibusan A , et al. A general approach to site-specific antibody drug conjugates[J]. Proc Natl Acad Sci USA, 2014,111(5):1766-1771. |
| [8] | Li X, Yang J, Rader C . Antibody conjugation via one and two C-terminal selenocysteines[J]. Methods, 2013,65(1):133-138. |
| [9] | Tang F, Yang Y, Tang Y , et al. One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody-drug conjugates[J]. Org Biomol Chem, 2016,14(40):9501-9518. |
| [10] | Dennler P, Fischer E, Schibli R . Antibody conjugates: from heterogeneous populations to defined reagents[J]. Antibodies, 2015,4(3):197-224. |
| [11] | Shen BQ, Xu K, Liu L , et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates[J]. Nat Biotechnol, 2012,30(2):184-189. |
| [12] | Wang L, Brock A, Schultz PG , et al. Expanding the genetic code of Escherichia coli[J]. Science, 2001,292(5516):498-500. |
| [13] | Slamon DJ, Leyland-Jones B, Shak B , et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med, 2001,344(11):783-792. |
| [14] | Meden H, Kuhn W . Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor[J]. Eur J Obstet Gynecol Reprod Biol, 1997,71(2):173-179. |
| [15] | Al-toub M, Vishnubalaji R, Hamam R , et al. CDH1 and IL1-beta expression dictates FAK and MAPKK-dependent cross-talk between cancer cells and human mesenchymal stem cells[J]. Stem Cell Res Ther, 2015,6(1):135. |
| [16] | Yang J, Yang G, Hou G , et al. Scutellaria barbata D. Don polysaccharides inhibit the growth of Calu-3 xenograft tumors via suppression of the HER2 pathway and angiogenesis[J]. Oncol Lett, 2015,9(6):2721-2725. |
| [17] | Nonagase Y, Yonesaka K, Kawakami H , et al. Heregulin-expressing HER2-positive breast and gastric cancer exhibited heterogeneous susceptibility to the anti-HER2 agents lapatinib, trastuzumab and T-DM1[J]. Oncotarget, 2016,7(51):84860-84871. |
| [18] | de Vlieghere E, Carlier C, Ceelen W , et al. Data on in vivo selection of SK-OV-3 luc ovarian cancer cells and intraperitoneal tumor formation with low inoculation numbers[J]. Data Brief, 2016,6:542-549. |
| [19] | Atnip AA, Sigurdson GT, Bomser J , et al. Time, concentration, and pH-dependent transport and uptake of anthocyanins in a human gastric epithelial (NCI-N87) cell line[J]. Int J Mol Sci, 2017,18(2):446. |
| [20] | Trail PA, Dubowchik GM, Lowinger TB . Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design[J]. Pharmacol Ther, 2018,181:126-142. |
| [21] | Behrens CR, Liu B . Methods for site-specific drug conjugation to antibodies[J]. MAbs, 2014,6(1):46-53. |
| [22] | Jain N, Smith SW, Ghone S , et al. Current ADC linker chemistry[J]. Pharm Res, 2015,32(11):3526-3540. |
| [23] | Diamantis N, Banerji U . Antibody-drug conjugates: an emerging class of cancer treatment[J]. Br J Cancer, 2016,114(4):362-367. |
| [24] | Panowski S, Bhakta S, Raab H , et al. Site-specific antibody drug conjugates for cancer therapy[J]. MAbs, 2014,6(1):34-45. |
| [25] | Fishkin N, Maloney EK, Chari RV , et al. A novel pathway for maytansinoid release from thioether linked antibody-drug conjugates (ADCs) under oxidative conditions[J]. Chem Commun, 2011,47(38):10752-10754. |
| [26] | Ponte JF, Sun X, Yoder NC , et al. Understanding how the stabi-lity of the thiol-maleimide linkage impacts the pharmacokinetics of lysine-linked antibody-maytansinoid conjugates[J]. Bioconjug Chem, 2016,27(7):1588-1598. |
| [1] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [2] | Yue WANG,Shuang ZHANG,Hong ZHANG,Li LIANG,Ling XU,Yuan-jia CHENG,Xue-ning DUAN,Yin-hua LIU,Ting LI. Clinicopathological features and prognosis of hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 853-862. |
|
||