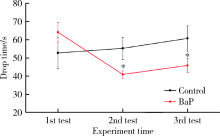
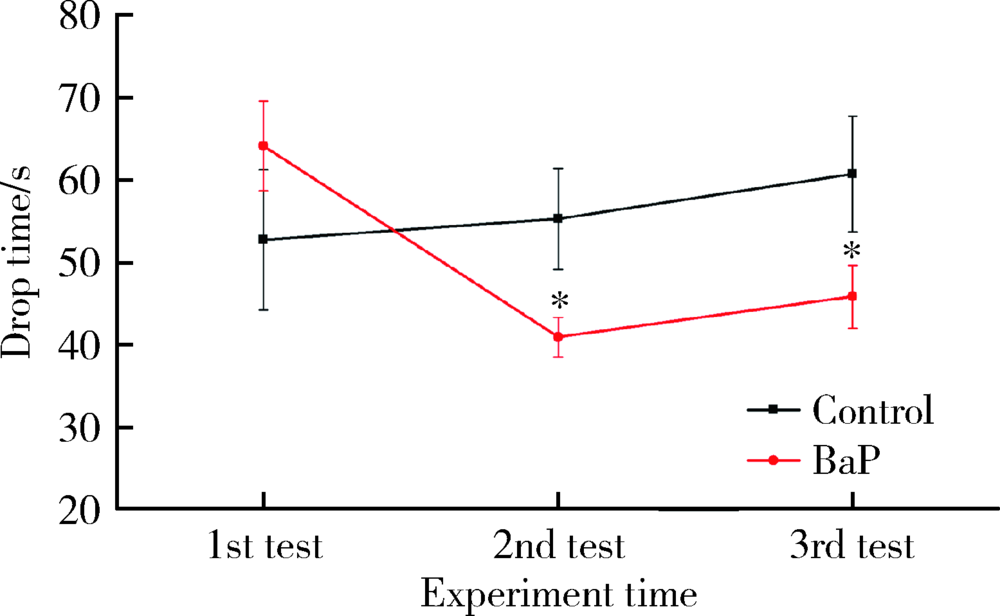
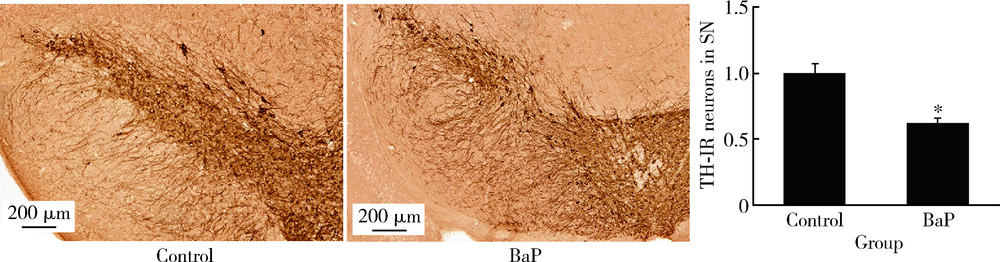
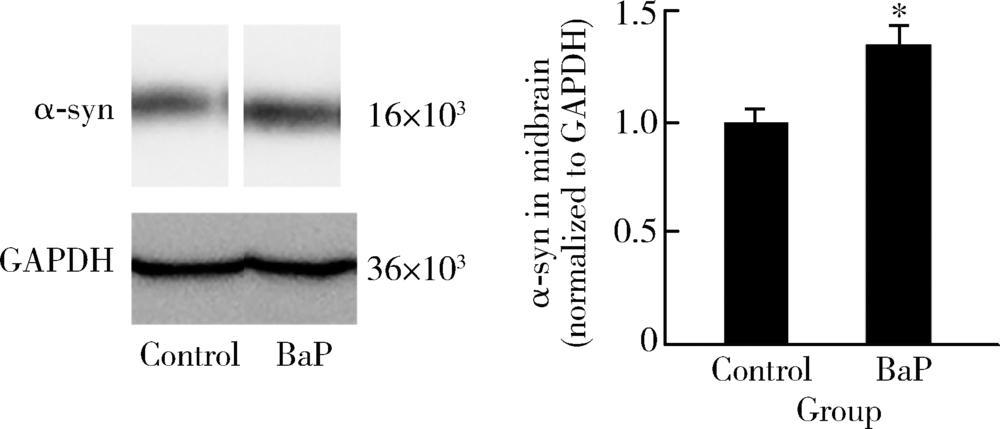
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 438-443. doi: 10.19723/j.issn.1671-167X.2020.03.007
Previous Articles Next Articles
Effect of benzo(a)pyrene on dopaminergic neurons and α-synuclein in brain and its mechanism involved
Yu-ze QI,Hui-hui QUAN,Wei-xing XU,Qing-ru LI,Hui ZHOU( )
)
- Department of Occupational and Environmental Health, Peking University School of Public Health, Beijing 100191, China
CLC Number:
- R12
| [1] |
Saunders C, Shockley D, Knuckles M. Behavioral effects induced by acute exposure to benzo(a)pyrene in F-344 rats[J]. Neurotox Res, 2001,3(6):557-579.
pmid: 15111245 |
| [2] |
Qiu CY, Cheng SQ, Xia YY, et al. Effects of subchronic benzo(a)pyrene exposure on neurotransmitter receptor gene expression in the rat hippocampus related with spatial learning and memory change[J]. Toxicology, 2011,289(2-3):83-90.
doi: 10.1016/j.tox.2011.07.012 pmid: 21839799 |
| [3] | Gao DX, Wu MF, Wang CG, et al. Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio)[J]. Aquat Toxicol, 2015,167(10):200-208. |
| [4] |
Burre J, Sharma M, Tsetsenis T, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro[J]. Science, 2010,329(5999):1663-1667.
doi: 10.1126/science.1195227 pmid: 20798282 |
| [5] | Naughton C, O’toole D, Kirik D, et al. Interaction between subclinical doses of the Parkinson’s disease associated gene, alpha-synuclein, and the pesticide, rotenone, precipitates motor dysfunction and nigrostriatal neurodegeneration in rats[J]. Behav Brain Res, 2017,316(1):160-168. |
| [6] | Wilson W, Shapiro L, Bradner J, et al. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring[J]. Neurotoxicology, 2014,44(9):279-287. |
| [7] |
Ali S, Rajini P. Elicitation of dopaminergic features of Parkinson’s disease in C. elegans by monocrotophos, an organophosphorous insecticide[J]. CNS Neurol Disord Drug Targets, 2012,11(8):993-1000.
doi: 10.2174/1871527311211080008 pmid: 23244418 |
| [8] | Palacios N, Fitzgerald K, Hart J, et al. Air pollution and risk of Parkinson's disease in a large prospective study of men[J]. Environ Health Perspect, 2017,125(8):1-7. |
| [9] | Lee P, Liu L, Sun Y, et al. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study[J]. Environ Int, 2016,96(11):75-81. |
| [10] |
Das M, Seth P, Mukhtar H. Distribution of benzo(a)pyrene in discrete regions of rat brain[J]. Bull Environ Contam Toxicol, 1985,35(4):500-504.
pmid: 4052652 |
| [11] | Michaelson J, Trump S, Rudzok S, et al. Transcriptional signatures of regulatory and toxic responses to benzo-[a]-pyrene exposure[J]. BMC Genomics, 2011,12(10):502-515. |
| [12] | Jayasekara S, Sharma R, Drown D. Effects of benzo[a]pyrene on steady-state levels of biogenic amines and metabolizing enzymes in mouse brain regions[J]. Ecotoxicol Environ Saf, 1992,24(1):1-12. |
| [13] | Naoi M, Maruyama W, Nagy G. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains[J]. Neurotoxicology, 2004,25(1-2):193-204. |
| [14] | Stephanou P, Konstandi M, Pappas P, et al. Alterations in central monoaminergic neurotransmission induced by polycyclic aromatic hydrocarbons in rats[J]. Eur J Drug Metab Pharmacokinet, 1998,23(4):475-481. |
| [15] | Bouayed J, Desor F, Rammal H, et al. Effects of lactational exposure to benzo[alpha]pyrene (B[alpha]P) on postnatal neurodevelopment, neuronal receptor gene expression and behaviour in mice[J]. Toxicology, 2009,259(3):97-106. |
| [16] |
Guillot T, Richardson J, Wang M, et al. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity[J]. Neuropeptides, 2008,42(4):423-434.
doi: 10.1016/j.npep.2008.04.003 |
| [17] | Giasson B, Duda J, Quinn S, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein[J]. Neuron, 2002,34(4):521-533. |
| [18] | Webb J, Ravikumar B, Atkins J, et al. Alpha-synuclein is degraded by both autophagy and the proteasome[J]. J Biol Chem, 2003,278(27):25009-25013. |
| [19] | Beilina A, Cookson M. Genes associated with Parkinson’s disease: Regulation of autophagy and beyond[J]. J Neurochem, 2016,139(10):91-107. |
| [20] | Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy[J]. PLoS One, 2010,5(2):e9313. |
| [1] | ZHANG Hui-feng, HUANG Huan-huan, ZHAO Yu-jia, LI Qing-ru, QI Yu-ze, ZHOU Hui. Effects of benzo(a)pyrene on expressions of insulin-degrading enzyme and neprilysin in neuroglia cells [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 401-407. |
|
||