Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (6): 1117-1123. doi: 10.19723/j.issn.1671-167X.2020.06.022
Previous Articles Next Articles
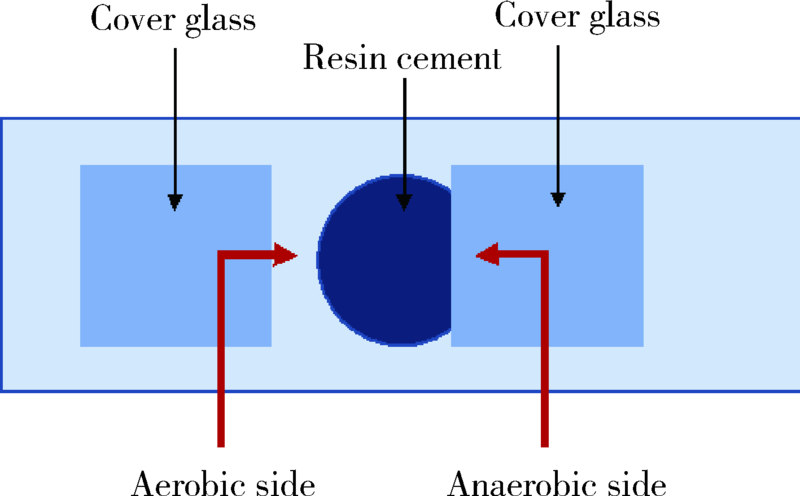
Curing method affecting the formation of oxygen inhibition layer on the surface of resin cement
Wen-xin CHEN,Xu-dong BAO( ),Lin YUE
),Lin YUE
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R783.1
| [1] |
Bergmann P, Noack MJ, Roulet JF. Marginal adaptation with glass-ceramic inlays adhesively luted with glycerine gel[J]. Quintessence Int, 1991,22(9):739-744.
pmid: 1946951 |
| [2] |
Gauthier MA, Stangel I, Ellis TH, et al. Oxygen inhibition in dental resins[J]. J Dent Res, 2005,84(8):725.
doi: 10.1177/154405910508400808 pmid: 16040730 |
| [3] |
Anirudhan TS, Rijith S. Synjournal and characterization of carboxyl terminated poly (methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium (Ⅵ) from aqueous media[J]. J Environ Radioactiv, 2012,106(2):8-19.
doi: 10.1016/j.jenvrad.2011.10.013 |
| [4] | 赵信义. 树脂基充填材料[M]//林红. 口腔材料学. 2版. 北京: 北京大学医学出版社, 2013: 143-144, 148-149. |
| [5] | 朱诚身. 透射电镜和扫描电镜//朱诚身. 聚合物结构分析[M]. 2版. 北京: 科学出版社, 2010: 476-480. |
| [6] | Schulz VG, Henrici G. Reaktionskinetik der polymerisationshemmung durch molekularen Sauerstoff[J]. Macromol Chem Phys, 1957,24(1):437-454. |
| [7] |
Yatabe M, Seki H, Shirasu N, et al. Effect of the reducing agent on the oxygen-inhibited layer of the cross-linked reline material[J]. J Oral Rehabil, 2001,28(2):180.
doi: 10.1046/j.1365-2842.2001.00634.x pmid: 11298268 |
| [8] | 林娇, 高诚辉, 郑开魁, 等. 稀土增强树脂基摩擦材料的摩擦磨损性能研究[J]. 润滑与密封, 2018,43(4):53-56. |
| [9] | 赵信义. 树脂基复合材料[M]//赵信义. 口腔材料学. 5版. 北京: 人民卫生出版社, 2012: 84-99. |
| [10] |
Bijelic-Donova J, Garoushi S, Lassila LV, et al. Oxygen inhibition layer of composite resins: effects of layer thickness and surface layer treatment on the interlayer bond strength[J]. Eur J Oral Sci, 2015,123(1):53-60.
doi: 10.1111/eos.12167 pmid: 25556290 |
| [11] |
Rahiotis C, Zinelis S, Elides T, et al. Setting characteristics of a resin infiltration system for incipient caries treatment[J]. J Dent, 2015,43(6):715-719.
doi: 10.1016/j.jdent.2015.03.010 pmid: 25862277 |
| [12] |
Courtecuisse F, Cerezo J, Croutxé-Barghorn C, et al. Depth characterization by confocal raman microscopy of oxygen inhibition in free radical photopolymerization of acrylates: Contribution of the thiol chemistry[J]. J Polym Sci Pol Chem, 2013,51(3):635-643.
doi: 10.1002/pola.26413 |
| [13] |
Truffier-Boutry D, Place E, Devaux J, et al. Interfacial layer characterization in dental composite[J]. J Oral Rehabil, 2010,30(1):74-77.
doi: 10.1046/j.1365-2842.2003.01008.x pmid: 12485387 |
| [14] |
Yamaji A, Koga K, Tsujimoto A, et al. Influence of oxygen-inhibited layer on dentin bond strength of chemical-cured resin composite[J]. Eur J Oral Sci, 2013,121(5):497-503.
pmid: 24028599 |
| [15] |
Souza RO, Mutlu O, Mesquita AM, et al. Effect of different polymerization devices on the degree of conversion and the physical properties of an indirect resin composite[J]. Acta Odontol Latinoam, 2010,23(2):129.
pmid: 21053686 |
| [16] |
Di Francescantonio M, Aguiac TR, Arrais CA, et al. Influence of viscosity and curing mode on degree of conversion of dual-cured resin cements[J]. Eur J Dent, 2013,7(1):81-85.
pmid: 23407604 |
| [17] |
Arrais CA, Giannini M, Rueggeberg FA. Kinetic analysis of monomer conversion in auto- and dual-polymerizing modes of commercial resin luting cements[J]. J Prosthet Dent, 2009,101(2):128-136.
doi: 10.1016/S0022-3913(09)60008-1 pmid: 19167537 |
| [18] |
Velazquez E, Vaidyanathan J, Vaidyanathan TK, et al. Effect of primer solvent and curing mode on dentin shear bond strength and interface morphology[J]. Quintessence Int, 2003,34(7):548.
pmid: 12946075 |
| [19] |
Giorgi MC, Aguiar FH, Soares LE, et al. Does an additional UV LED improve the degree of conversion and Knoop Hardness of light-shade composite resins[J]. Eur J Dent, 2012,6(4):396-401.
pmid: 23077419 |
| [20] |
Rastelli AN, Jacomassi DP, Bagnato VS. Effect of power densities and irradiation times on the degree of conversion and temperature increase of a microhybrid dental composite resin[J]. Laser Phys, 2008,18(9):1074-1079.
doi: 10.1134/S1054660X08090132 |
| [21] | Nicoleta I. Comparative effect of self- or dual-curing on polymerization kinetics and mechanical properties in a novel, dental-resin-based composite with alkaline filler. running title: resin-com-posites with alkaline fillers[J]. Mater, 2018,11(1):108. |
| [22] | 中华口腔医学会牙体牙髓病学专业委员会. 复合树脂直接粘接修复中光固化灯使用及操作规范的专家共识[J]. 中华口腔医学杂志, 2018,53(9):579-584. |
| [23] |
Hannig M, Bott B. In-vitro pulp chamber temperature rise during composite resin polymerization with various light-curing sources[J]. Dent Mater, 1999,15(4):275-281.
doi: 10.1016/s0109-5641(99)00047-0 pmid: 10551096 |
| [24] |
Kim MJ, Kim RJ, Ferracane J, et al. Thermographic analysis of the effect of composite type, layering method, and curing light on the temperature rise of photo-cured composites in tooth cavities[J]. Dent Mater, 2017,33(10):e373.
doi: 10.1016/j.dental.2017.07.007 pmid: 28760330 |
| [25] |
Yatabe M, Yasuda N, Ai M, et al. Unpolymerized Layer on Autopolymerizing, Hard Reline Materials[J]. Int J Prosthodont, 1999,12(2):129.
pmid: 10371914 |
| [26] | Park HH, Lee IB. Effect of glycerin on the surface hardness of composites after curing[J]. J Korean Acad Cons Dent, 2011,36(6):483-488. |
| [1] | Yi-xiang PAN,Xiu-hua LI,Fu-cong TIAN,Xiao-yan WANG. Effect of intrapulpal pressure on the bonding strength of resin cement to dentin [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 321-326. |
| [2] | LIAO Yu, LIU Xiao-qiang, CHEN Li, ZHOU Jian-feng, TAN Jian-guo. Effects of different surface treatments on the zirconia-resin cement bond strength [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 53-57. |
| [3] | FU Zhao-ran,TIAN Fu-cong,ZHANG Lu,HAN Bing,WANG Xiao-yan. Curing mode of universal adhesives affects the bond strength of resin cements to dentin [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 101-104. |
| [4] | 吕Pin , JIANG Ting. Effect of surface hot-etching treatment on zirconia/resin bonding strength [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 302-305. |
|
||