Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (4): 705-711. doi: 10.19723/j.issn.1671-167X.2022.04.020
Previous Articles Next Articles
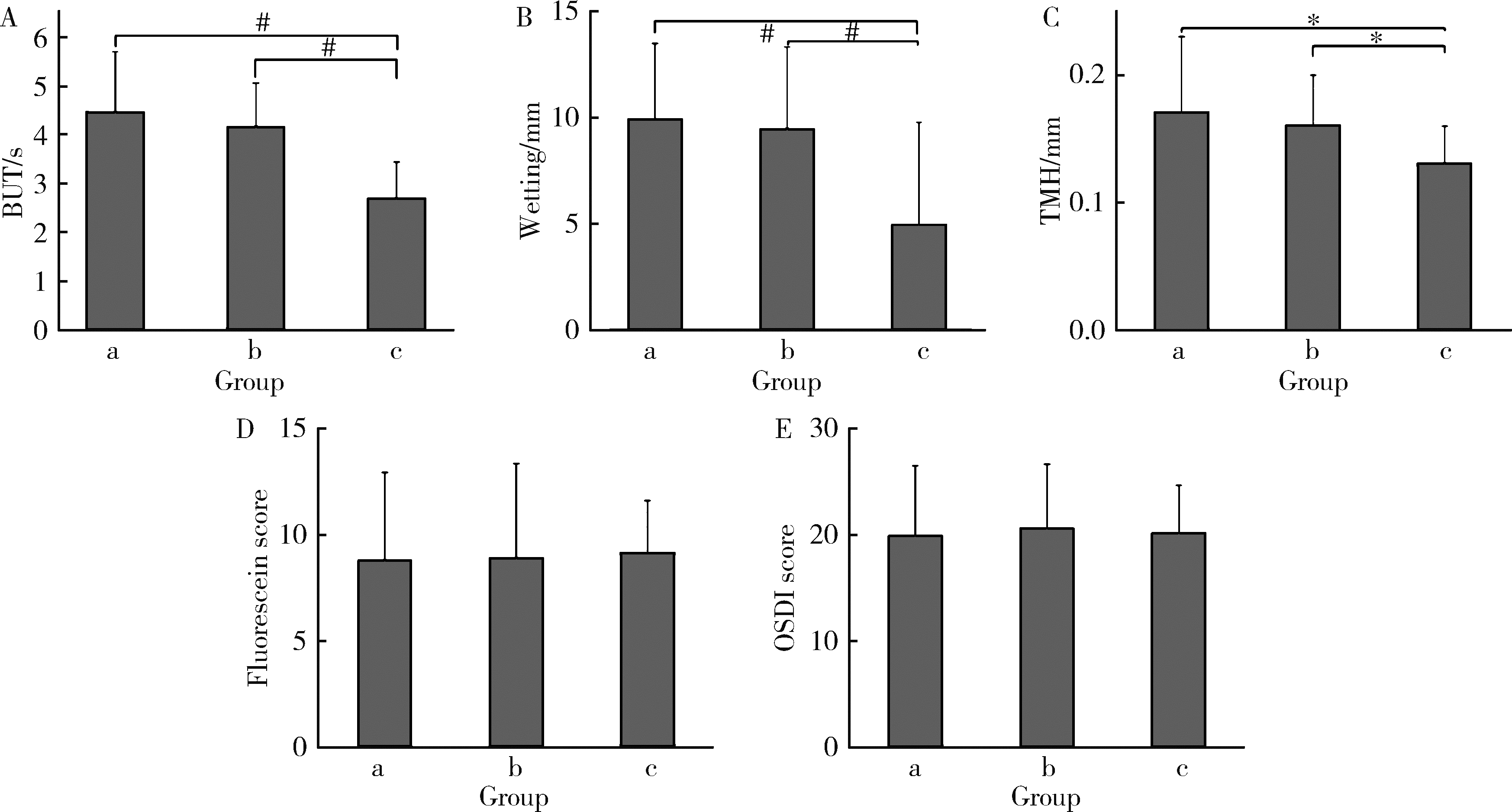
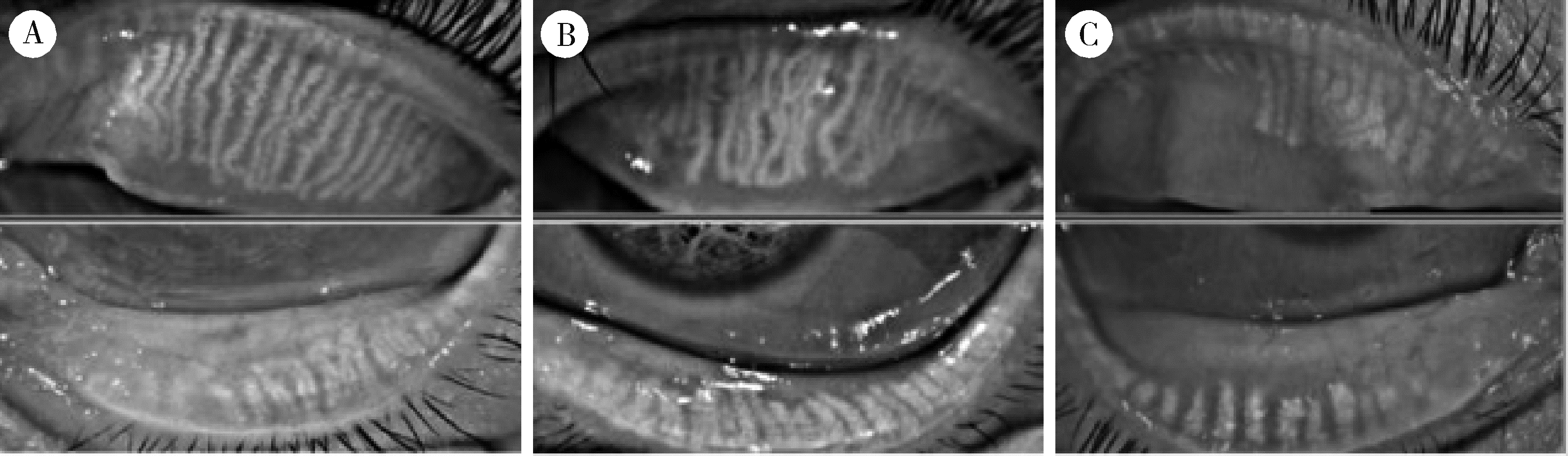
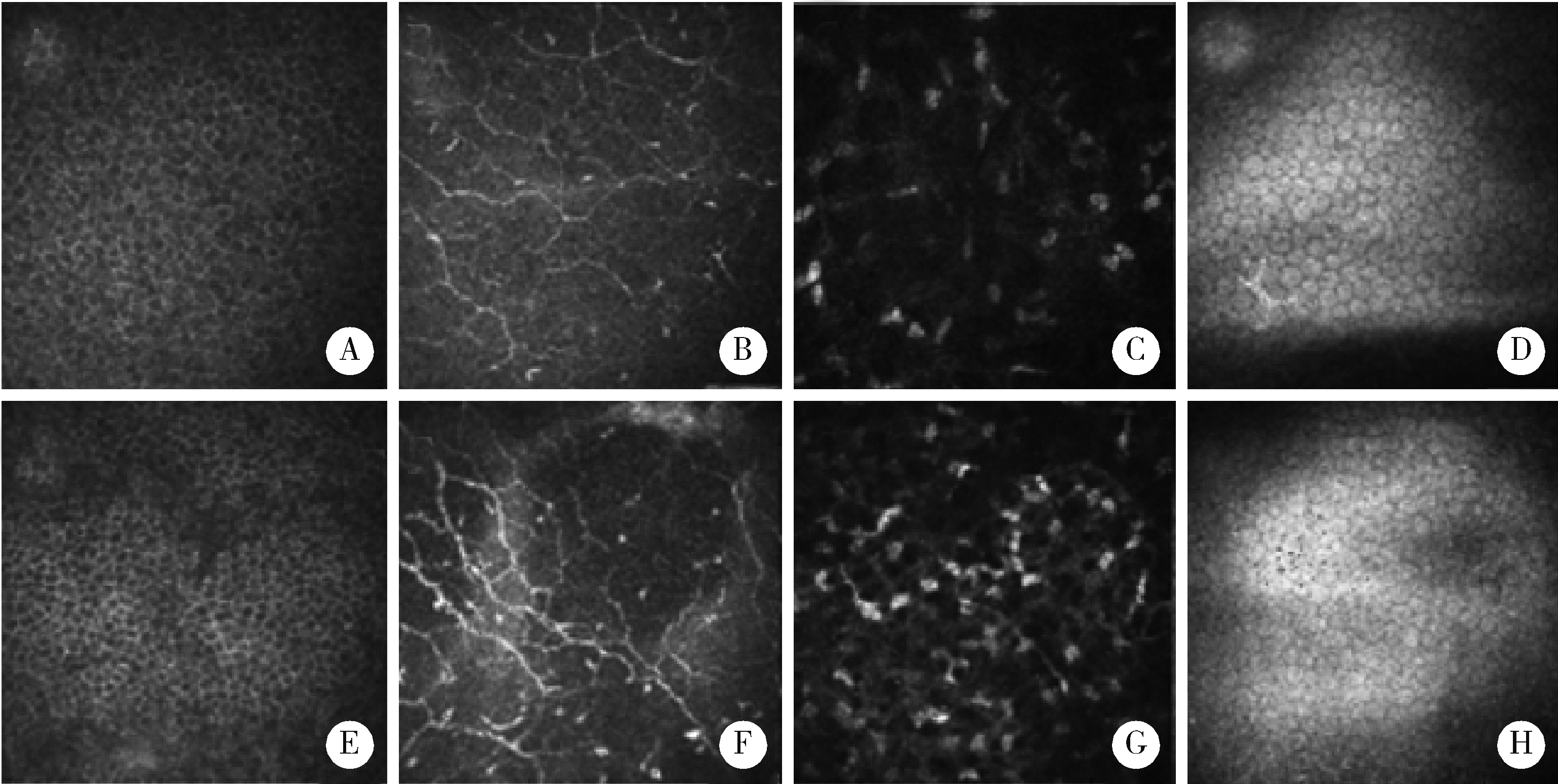
Evaluation of ocular surface status and function in primary Sjögren's syndrome with hypothyroidism
Hao-zhe YU1,Wei-zhen ZENG1,Wen-yu WU1,Zhong-qiang YAO2,*( ),Yun FENG1,*(
),Yun FENG1,*( )
)
- 1. Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
2. Department of Rheumatology and Immunology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R593.2
| 1 | Chowdhury F, Tappuni A, Bombardieri M. Biological therapy in primary Sjögren's syndrome: Effect on salivary gland function and inflammation[J/OL]. Front Med, 2021, 8: 707104[2021-07-01]. https://www.frontiersin.org/articles/10.3389/fmed.2021.707104/full. |
| 2 | Bron AJ , de Paiva CS , Chauhan SK , et al. TFOS DEWS Ⅱ pathophysiology report[J]. Ocul Surf, 2017, 15 (3): 438- 510. |
| 3 |
Villarreal-Gonzalez AJ , Rivera-Alvarado IJ , Rodriguez-Gutierrez LA , et al. Analysis of ocular surface damage and visual impact in patients with primary and secondary Sjögren syndrome[J]. Rheumatol Int, 2020, 40 (8): 1249- 1257.
doi: 10.1007/s00296-020-04568-7 |
| 4 |
Xu D , Zhao S , Li Q , et al. Characteristics of Chinese patients with primary Sjögren's syndrome: Preliminary report of a multi-centre registration study[J]. Lupus, 2020, 29 (1): 45- 51.
doi: 10.1177/0961203319889666 |
| 5 |
Wang TJ , Wang IJ , Hu CC , et al. Comorbidities of dry eye disease: A nationwide population-based study[J]. Acta Ophthalmol, 2012, 90 (7): 663- 668.
doi: 10.1111/j.1755-3768.2010.01993.x |
| 6 |
亚洲干眼协会中国分会, 海峡两岸医药卫生交流协会眼科学专业委员会眼表与泪液病学组中国医师协会眼科医师分会眼表与干眼学组. 中国干眼专家共识: 检查和诊断(2020年)[J]. 中华眼科杂志, 2020, 56 (10): 741- 747.
doi: 10.3760/cma.j.cn112142-20200714-00477 |
| 7 |
Shiboski CH , Shiboski SC , Seror R , et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts[J]. Arthritis Rheumatol, 2017, 69 (1): 35- 45.
doi: 10.1002/art.39859 |
| 8 |
中华医学会内分泌学分会. 成人甲状腺功能减退症诊治指南[J]. 中华内分泌代谢杂志, 2017, 33 (2): 167- 180.
doi: 10.3760/cma.j.issn.1000-6699.2017.02.018 |
| 9 |
Oliveira-Soto L , Efron N . Morphology of corneal nerves using confocal microscopy[J]. Cornea, 2001, 20 (4): 374- 384.
doi: 10.1097/00003226-200105000-00008 |
| 10 | Sun X, Lu L, Li Y, et al. Increased risk of thyroid disease in patients with Sjögren's syndrome: A systematic review and meta-analysis[J/OL]. Peerj, 2019, 7: 6737[2021-07-01]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6430100/. |
| 11 |
Sepulveda JIR , Kvarnstrom M , Eriksson P , et al. Long-term follow-up in primary Sjögren's syndrome reveals differences in clinical presentation between female and male patients[J]. Biol Sex Differ, 2017, 8 (1): 25.
doi: 10.1186/s13293-017-0146-6 |
| 12 |
Jara LJ , Navarro C , Brito-Zerón MDP , et al. Thyroid disease in Sjögren's syndrome[J]. Clin Rheumatol, 2007, 26 (10): 1601- 1606.
doi: 10.1007/s10067-007-0638-6 |
| 13 |
Williams DL , Pierce V , Mellor P , et al. Reduced tear production in three canine endocrinopathies[J]. J Small Anim Pract, 2007, 48 (5): 252- 256.
doi: 10.1111/j.1748-5827.2007.00349.x |
| 14 |
Dias AC , MóDulo CM , Jorge ALG , et al. Influence of thyroid hormone on thyroid hormone receptor β-1 expression and lacrimal gland and ocular surface morphology[J]. Invest Ophth Vis Sci, 2007, 48 (7): 3038- 3042.
doi: 10.1167/iovs.06-1309 |
| 15 |
Kang YS , Lee HS , Li Y , et al. Manifestation of meibomian gland dysfunction in patients with Sjögren's syndrome, non-Sjögren's dry eye, and non-dry eye controls[J]. Int Ophthalmol, 2018, 38 (3): 1161- 1167.
doi: 10.1007/s10792-017-0577-4 |
| 16 |
Wang LX , Deng YP . Androgen and meibomian gland dysfunction: From basic molecular biology to clinical applications[J]. Int J Ophthalmol, 2021, 14 (6): 915- 922.
doi: 10.18240/ijo.2021.06.18 |
| 17 |
Satitpitakul V , Rattanaphong T , Pruksakorn V . Meibomian glands dropout in patients with inactive thyroid related orbitopathy[J]. PLoS One, 2021, 16 (4): e0250617.
doi: 10.1371/journal.pone.0250617 |
| 18 | Altay M , Şahin T , Yildiz Z , et al. Changes in conjunctiva morphology using impression cytology in patients with Hashimoto's thyroiditis without thyroid-associated ophthalmopathy[J]. Turk Patoloji Derg, 2019, 35 (3): 213- 220. |
| 19 |
Wang Q , Shangguan J , Zhang Y , et al. The prevalence of thyroid autoantibodies in autoimmune connective tissue diseases: A systematic review and meta-analysis[J]. Expert Rev Clin Immunol, 2020, 16 (9): 923- 930.
doi: 10.1080/1744666X.2020.1811089 |
| 20 | 解如山. 桥本甲状腺炎患者发生干眼症的相关联性分析[J]. 现代医药卫生, 2015, 31 (22): 3436- 3438. |
| 21 | Altin Ekin M , Karadeniz Ugurlu S , Egrilmez ED , et al. Ocular surface changes in Hashimoto's thyroiditis without thyroid ophthalmopathy[J]. Eye Contact Lens, 2021, 47 (1): 32- 37. |
| 22 | Xu J, Chen P, Yu C, et al. In vivo confocal microscopic evaluation of corneal dendritic cell density and subbasal nerve parameters in dry eye patients: A systematic review and meta-analysis[J/OL]. Front Med, 2021, 8: 578233[2021-07-01]. https://www.frontiersin.org/articles/10.3389/fmed.2021.578233/full. |
| 23 | Li F, Zhang Q, Ying X, et al. Corneal nerve structure in patients with primary Sjögren's syndrome in China[J/OL]. BMC Ophthalmol, 2021, 21(1): 211[2021-07-01]. https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-021-01967-7. |
| 24 | Gabbriellini G , Baldini C , Varanini V , et al. In vivo confocal scanning laser microscopy in patients with primary Sjögren's syndrome: A monocentric experience[J]. Mod Rheumatol, 2015, 25 (4): 585- 589. |
| 25 | Levine H, Hwang J, Dermer H, et al. Relationships between activated dendritic cells and dry eye symptoms and signs[J/OL]. Ocul Surf, 2021, 21: 186-192[2021-07-01]. https://www.sciencedirect.com/science/article/pii/S1542012421000513?via%3Dihub. |
| 26 | Cardigos J , Barcelos F , Carvalho H , et al. Tear meniscus and corneal sub-basal nerve plexus assessment in primary Sjögren syndrome and sicca syndrome patients[J]. Cornea, 2019, 38 (2): 221- 228. |
| [1] | CAI Jia, ZHANG Man. Variation tendency in serum high density lipoprotein cholesterol and apolipopro-tein A-Ⅰ in different thyroid function status during pregnancy [J]. Journal of Peking University(Health Sciences), 2015, 47(6): 910-913. |
|
||