Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (5): 1038-1046. doi: 10.19723/j.issn.1671-167X.2022.05.034
Previous Articles Next Articles
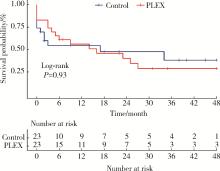
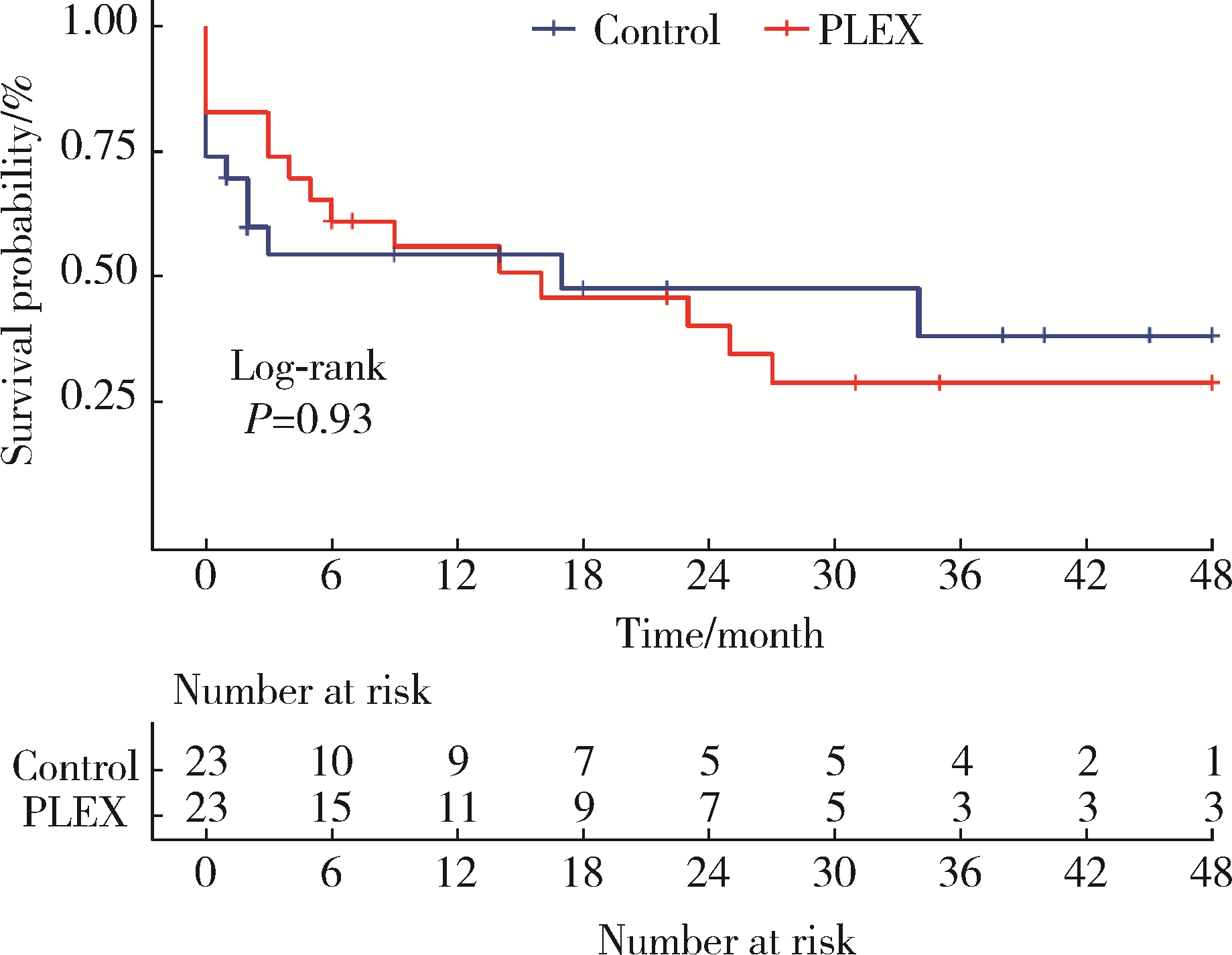
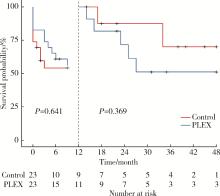
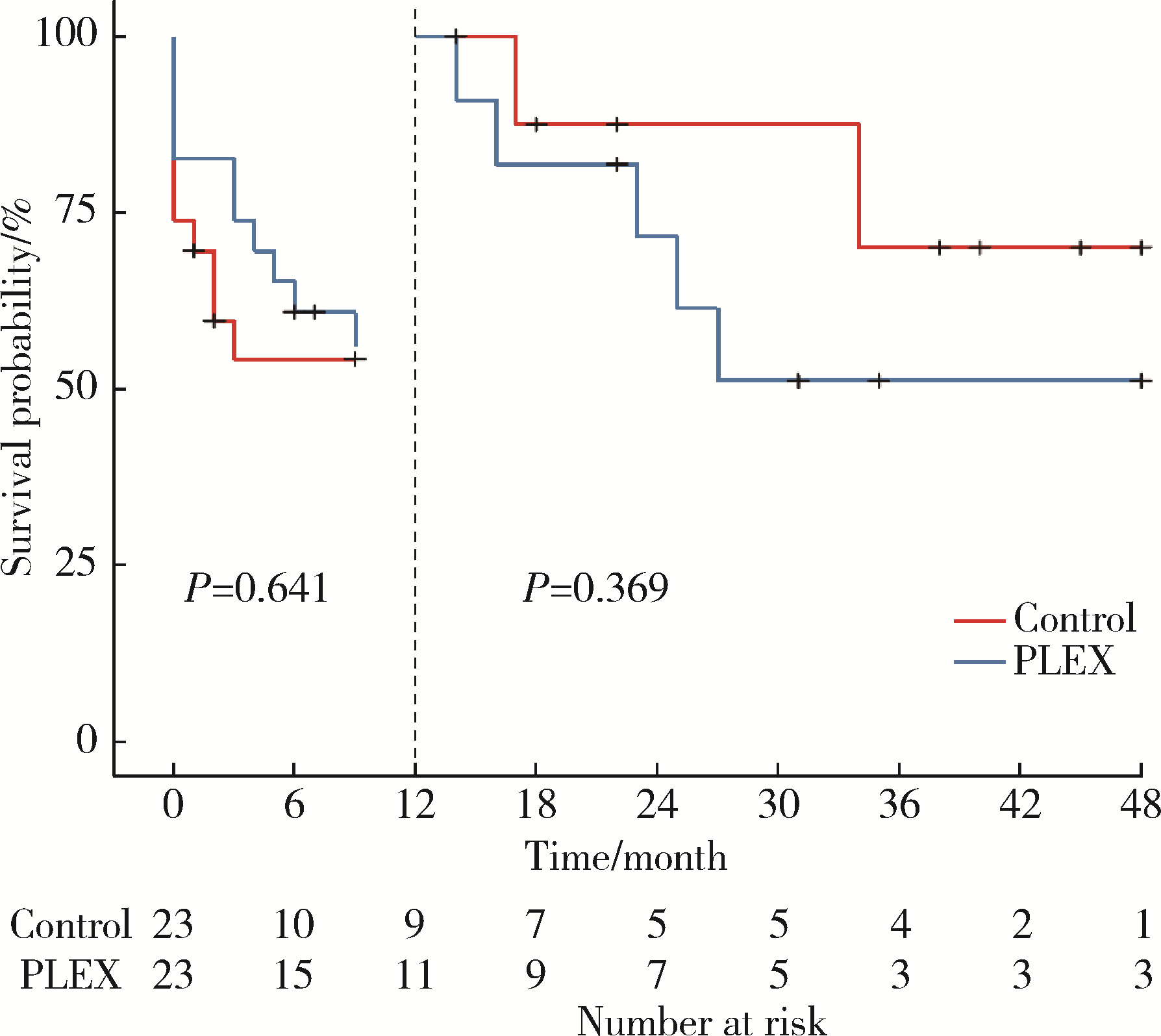
Efficacy of plasma exchange in severe crescentic IgA nephropathy: A multicentered, cohort study
Zi WANG1,Jun-jun ZHANG2,Li ZUO3,Yue WANG4,Wen-ge LI5,Hong CHENG6,Guang-yan CAI7,Hua-ying PEI8,Li-hua WANG9,Xu-jie ZHOU1,Su-fang SHI1,Li-jun LIU1,Ji-cheng LV1,*( ),Hong ZHANG1
),Hong ZHANG1
- 1. Renal Division, Peking University First Hospital; Institute of Nephrology, Peking University; Key Laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of CKD Prevention and Treatment, Ministry of Education of China, Research Units of Diagnosis and Treatment of lmmune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing 100191, China
2. Department of Nephrology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
3. Department of Nephrology, Peking University People's Hospital, Beijing 100044, China
4. Department of Nephrology, Peking University Third Hospital, Beijing 100191, China
5. Department of Nephrology, China-Japan Friendship Hospital, Beijing 100029, China
6. Division of Nephrology, Beijing Anzhen Hospital, Capital Medical University, Beijing 10029, China
7. Department of Nephrology, Chinese PLA General Hospital, Beijing 100853, China
8. Renal Division, Department of Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, China
9. Renal Division, Shanxi Medical University Second Affiliated Hospital, Taiyuan 030001, China
CLC Number:
- R692
| 1 |
Cattran DC , Feehally J , Cook HT , et al. Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis[J]. Kidney Int, 2012, 2 (2): 139- 274.
doi: 10.1038/kisup.2012.9 |
| 2 | Kidney disease: Improving global outcomes (KDIGO) glomerular diseases work group . KDIGO 2021 Clinical practice guideline for the management of glomerular diseases[J]. Kidney Int, 2021, 100 (Suppl 4): S1- S276. |
| 3 |
Lv J , Yang Y , Zhang H , et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study[J]. J Am Soc Nephrol, 2013, 24 (12): 2118- 2125.
doi: 10.1681/ASN.2012101017 |
| 4 |
Le vy , Jeremy B . Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression[J]. Ann Intern Med, 2001, 134 (11): 1033- 1042.
doi: 10.7326/0003-4819-134-11-200106050-00009 |
| 5 |
Jayne DR , Gaskin G , Rasmussen N , et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis[J]. J Am Soc Nephrol, 2007, 18 (7): 2180- 2188.
doi: 10.1681/ASN.2007010090 |
| 6 |
Suzuki H , Kiryluk K , Novak J , et al. The Pathophysiology of IgA nephropathy[J]. J Am Soc Nephrol, 2011, 22 (10): 1795- 1803.
doi: 10.1681/ASN.2011050464 |
| 7 |
Chambers ME , McDonald BR , Hall FW , et al. Plasmapheresis for crescentic IgA nephropathy: A report of two cases and review of the literature[J]. J Clin Apher, 1999, 14 (4): 185- 187.
doi: 10.1002/(SICI)1098-1101(1999)14:4<185::AID-JCA6>3.0.CO;2-K |
| 8 | Roccatello D , Ferro M , Coppo R , et al. Report on intensive treatment of extracapillary glomerulonephritis with focus on cresentic IgA nephropathy[J]. Nephrol Dial Transplant, 1995, 10 (11): 2054- 2059. |
| 9 |
Fujinaga S , Ohtomo Y , Umino D , et al. Plasma exchange combined with immunosuppressive treatment in a child with rapidly progressive IgA nephropathy[J]. Pediatr Nephrol, 2007, 22 (6): 899- 902.
doi: 10.1007/s00467-006-0428-4 |
| 10 |
Xie X , Lv J , Shi S , et al. Plasma exchange as an adjunctive therapy for crescentic IgA nephropathy[J]. Am J Nephrol, 2016, 44 (2): 141- 149.
doi: 10.1159/000448767 |
| 11 |
Padmanabhan A , Connelly-Smith L , Aqui N , et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence based approach from the writing committee of the american society for apheresis: The eighth special issue[J]. J Clin Apher, 2019, 34 (3): 171- 354.
doi: 10.1002/jca.21705 |
| 12 |
Wyatt RJ , Julian BA . IgA nephropathy[J]. N Engl J Med, 2013, 368 (25): 2402- 2414.
doi: 10.1056/NEJMra1206793 |
| 13 |
Yu G , Zhang Y , Meng B , et al. O-glycoforms of polymeric immunoglobulin A1 in the plasma of patients with IgA nephropathy are associated with pathological phenotypes[J]. Nephrol Dial Transplant, 2021, 37 (1): 33- 41.
doi: 10.1093/ndt/gfab204 |
| 14 | Yamazaki Y , Mori A , Nomura Y , et al. A case of rapidly progressive IgA nephropathy with transient hypocomplementemia at onset[J]. Nihon Jinzo Gakkai Shi, 1997, 39 (7): 765- 770. |
| 15 | Coppo R , Basolo B , Roccatello D , et al. Immunological monitoring of plasma exchange in primary IgA nephropathy[J]. Artif Organs, 2010, 9 (4): 351- 360. |
| 16 |
Lai KN , Lai FM , Leung AC , et al. Plasma exchange in patients with rapidly progressive idiopathic IgA nephropathy: A report of two cases and review of literature[J]. Am J Kidney Dis, 1987, 10 (1): 66- 70.
doi: 10.1016/S0272-6386(87)80014-8 |
| 17 | Wang Z, Jiang Y, Chen P, et al. The level of urinary C4d is associated with disease progression in IgA nephropathy with glomerular crescentic lesions: A cohort study[J]. Nephrol Dial Transplant, 2022[2022-06-01]. https://academic.oup.com/ndt/advance-article-abstract/doi/10.1093/ndt/gfac024/6519273?redirectedFrom=fulltext&login=false |
| 18 |
Rosenblad T , Rebetz J , Johansson M , et al. Eculizumab treatment for rescue of renal function in IgA nephropathy[J]. Pediatr Nephrol, 2014, 29 (11): 2225- 2228.
doi: 10.1007/s00467-014-2863-y |
| 19 |
Troels R , Bang P B , Giedrius S , et al. Use of eculizumab in crescentic IgA nephropathy: Proof of principle and conundrum[J]. Clin Kidney J, 2015, 8 (5): 489- 491.
doi: 10.1093/ckj/sfv076 |
| 20 | Matsumura D , Tanaka A , Nakamura T , et al. Coexistence of atypical hemolytic uremic syndrome and crescentic IgA nephropathy treated with eculizumab: A case report[J]. Clin Nephrol Case Stud, 2016, 4, 24- 28. |
| 21 |
Lafayette RA , Rovin BH , Reich HN , et al. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy[J]. Kidney Int Rep, 2020, 5 (11): 2032- 2041.
doi: 10.1016/j.ekir.2020.08.003 |
| [1] | Wen-han BAO,Wen TANG. Changes of gut microflora in newly diagnosed IgA nephropathy patients and its correlation with clinical risk factors [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 124-132. |
| [2] | Yu-qi KANG,Yue-miao ZHANG,Ping HOU,Su-fang SHI,Li-jun LIU,Xu-jie ZHOU,Ji-cheng LV,Hong ZHANG. Trans-ethnic analysis of susceptibility variants in IgA nephropathy [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 459-466. |
| [3] | WU Gang, PENG You-ming, XU Dao-liang, LIU Chang-hua. Abnormal percentage of memory B cells in tonsils and peripheral blood in clinical progression of IgA nephropathy [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 749-753. |
|
||