Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (5): 907-919. doi: 10.19723/j.issn.1671-167X.2022.05.018
Previous Articles Next Articles
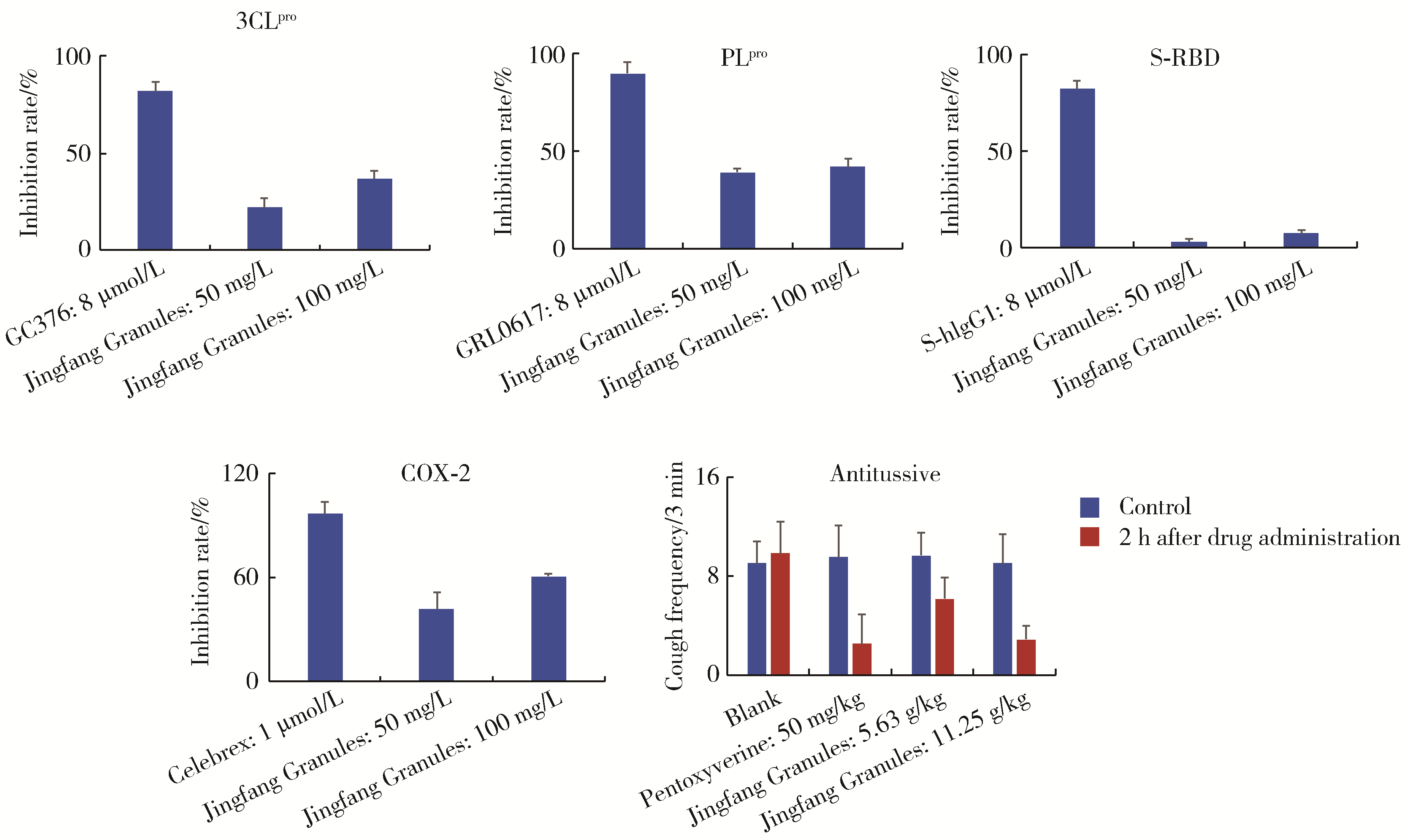
Bioactive compounds of Jingfang Granules against SARS-CoV-2 virus proteases 3CLpro and PLpro
Zhan-peng SHANG,Yang YI,Rong YU,Jing-jing FAN,Yu-xi HUANG,Xue QIAO,Min YE*( )
)
- State Key Laboratory of Natural and Biomimetic Drugs, Peking University-Yunnan Baiyao International Medical Research Center, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China
CLC Number:
- R932
| 1 |
Huang C , Wang Y , Li X , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395 (10223): 497- 506.
doi: 10.1016/S0140-6736(20)30183-5 |
| 2 | World Health Organization. WHO coronavirus (COVID-19) dashboard[EB/OL]. (2022-04-29)[2022-05-01]. https://covid19.who.int. 2021. |
| 3 | Merck. Merck and ridgeback's molnupiravir, an oral COVID-19 antiviral medicine, receives first authorization in the world[EB/OL]. (2021-11-04)[2022-04-15]. https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-an-oral-covid-19-antiviral-medicine-receives-first-authorization-in-the-world. 2021. |
| 4 | Pfizer. Pfizer to provide U.S. government with 10 million treatment courses of investigational oral antiviral candidate to help combat COVID-19[EB/OL]. (2021-11-18)[2022-04-18]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provide-us-government-10-million-treatment-courses.html. 2021. |
| 5 |
Dai W , Zhang B , Jiang X , et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease[J]. Science, 2020, 368 (6497): 1331- 1335.
doi: 10.1126/science.abb4489 |
| 6 |
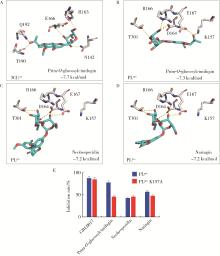
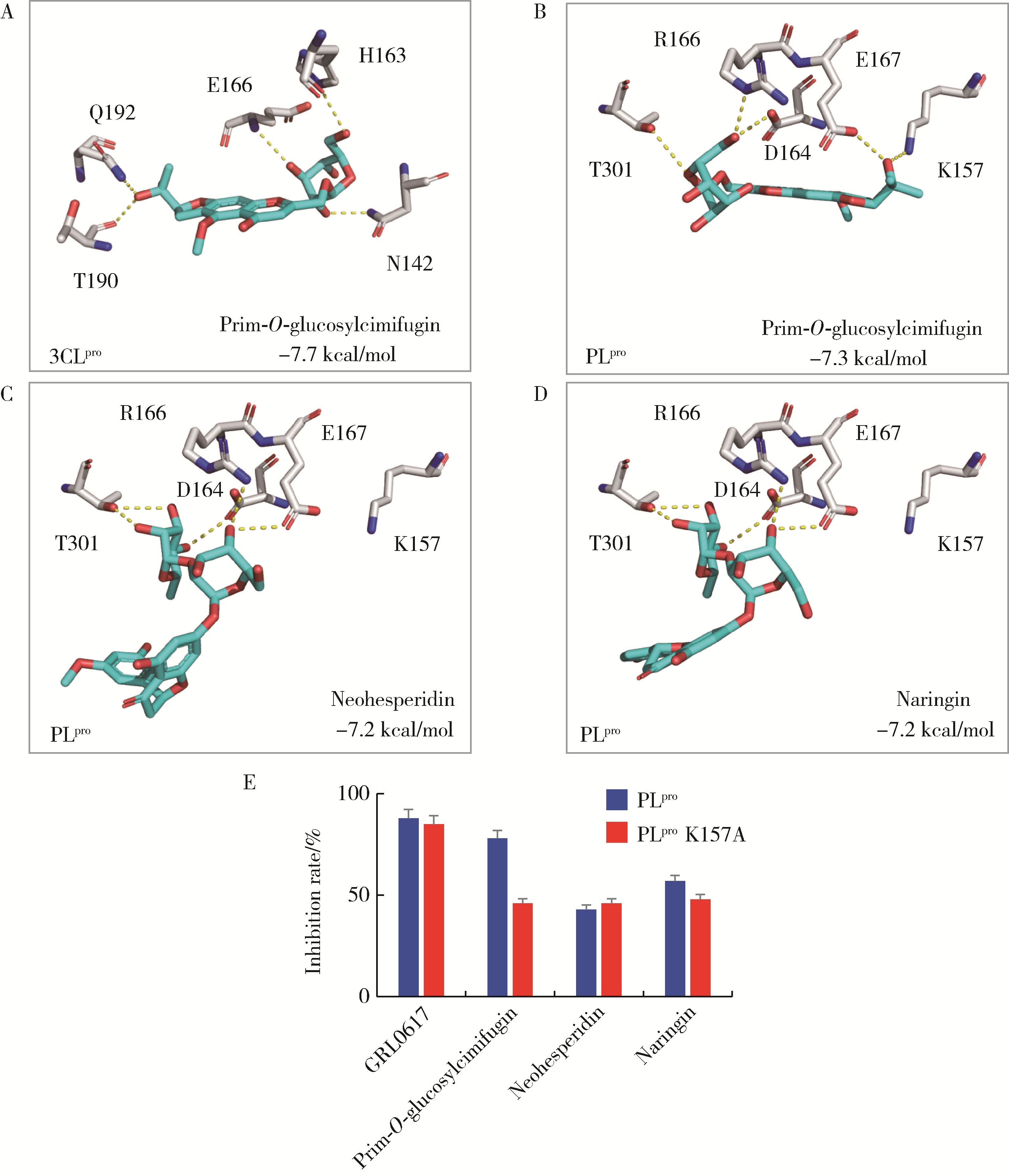
Fu Z , Huang B , Tang J , et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery[J]. Nat Commun, 2021, 12 (1): 488.
doi: 10.1038/s41467-020-20718-8 |
| 7 |
付新, 刘阳, 王雪梅, 等. 麻杏石甘汤的研究进展[J]. 中医药信息, 2017, 34 (2): 126- 128.
doi: 10.3969/j.issn.1002-2406.2017.02.037 |
| 8 |
赵琰, 胡杰, 张贵民, 等. 荆防败毒散的源流与应用[J]. 环球中医药, 2020, 13 (11): 1996- 2002.
doi: 10.3969/j.issn.1674-1749.2020.11.042 |
| 9 | 冯芹, 张贵民. 荆防败毒散治疗急性呼吸道感染的临床应用以及作用机制的探讨[J]. 中药与临床, 2020, 11 (3): 28- 32. |
| 10 | 邹胜. 荆防败毒散治疗急性病毒性上呼吸道感染[J]. 山西中医, 2010, 26 (3): 11- 12. |
| 11 |
殷健操, 周荣, 黄仁礼. 从"热入血室"论治新型冠状病毒肺炎产妇1例[J]. 光明中医, 2020, 35 (16): 2560- 2562.
doi: 10.3969/j.issn.1003-8914.2020.16.047 |
| 12 | 张奎, 陈红英, 马瑜. 荆防败毒散药效学研究[J]. 河南中医, 2009, 29 (6): 601- 602. |
| 13 | Song Y , Jing W , Yan R , et al. Research progress of the studies on the roots of Peucedanum praeruptorum dunn (Peucedani Radix)[J]. Pak J Pharm Sci, 2015, 28 (1): 71- 81. |
| 14 | Xi J , Xiang S , Zhang H , et al. Clinical observation of arbidol combined with diammonium glycyrrhizinate in the treatment of COVID-19[J]. Chin J Hosp Phar, 2020, 40 (12): 1287- 1290. |
| 15 |
Shi R , Xu JW , Xiao ZT , et al. Naringin and naringenin relax rat tracheal smooth by regulating BKCa activation[J]. J Med Food, 2019, 22 (9): 963- 970.
doi: 10.1089/jmf.2018.4364 |
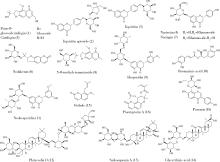
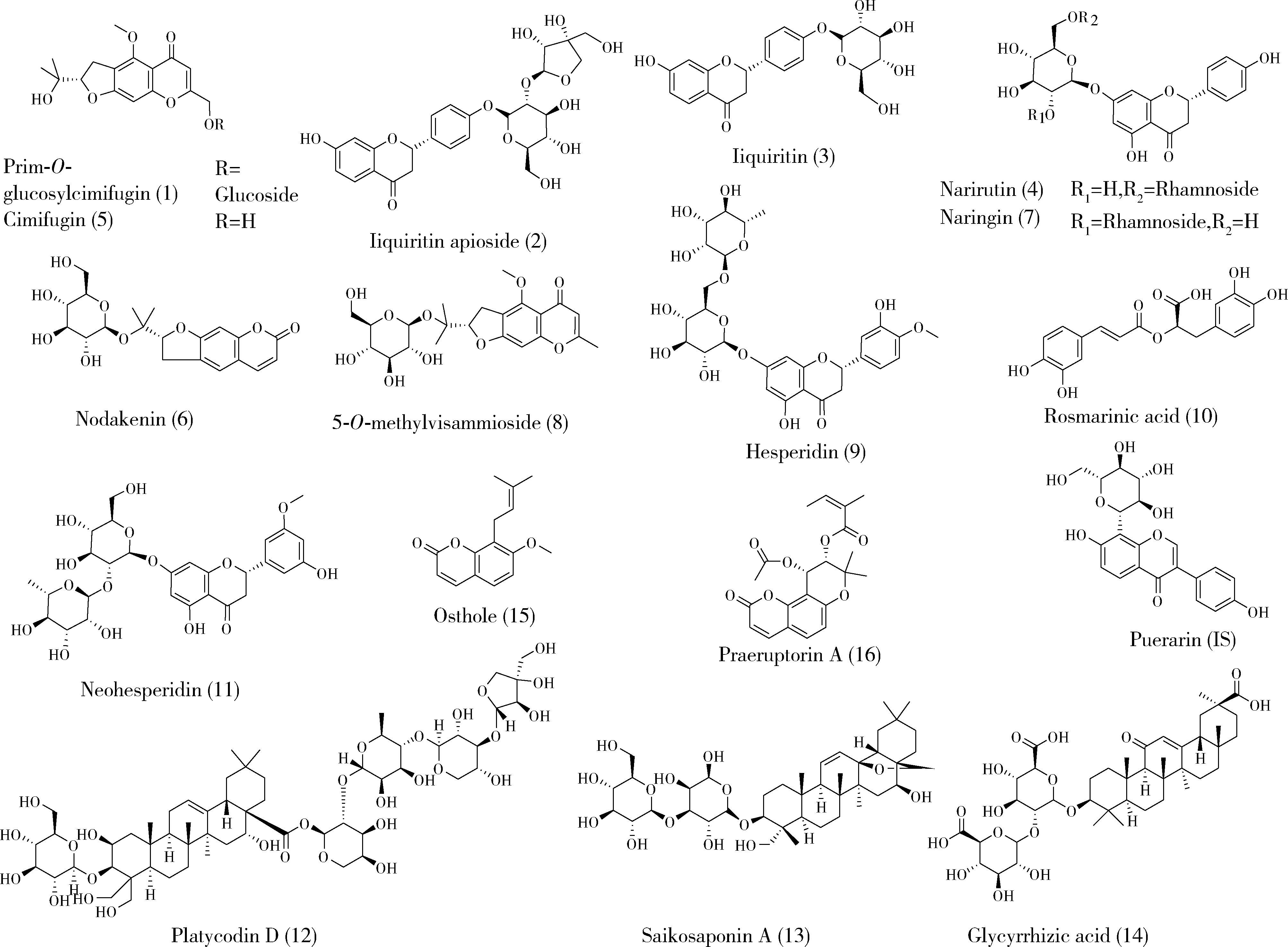
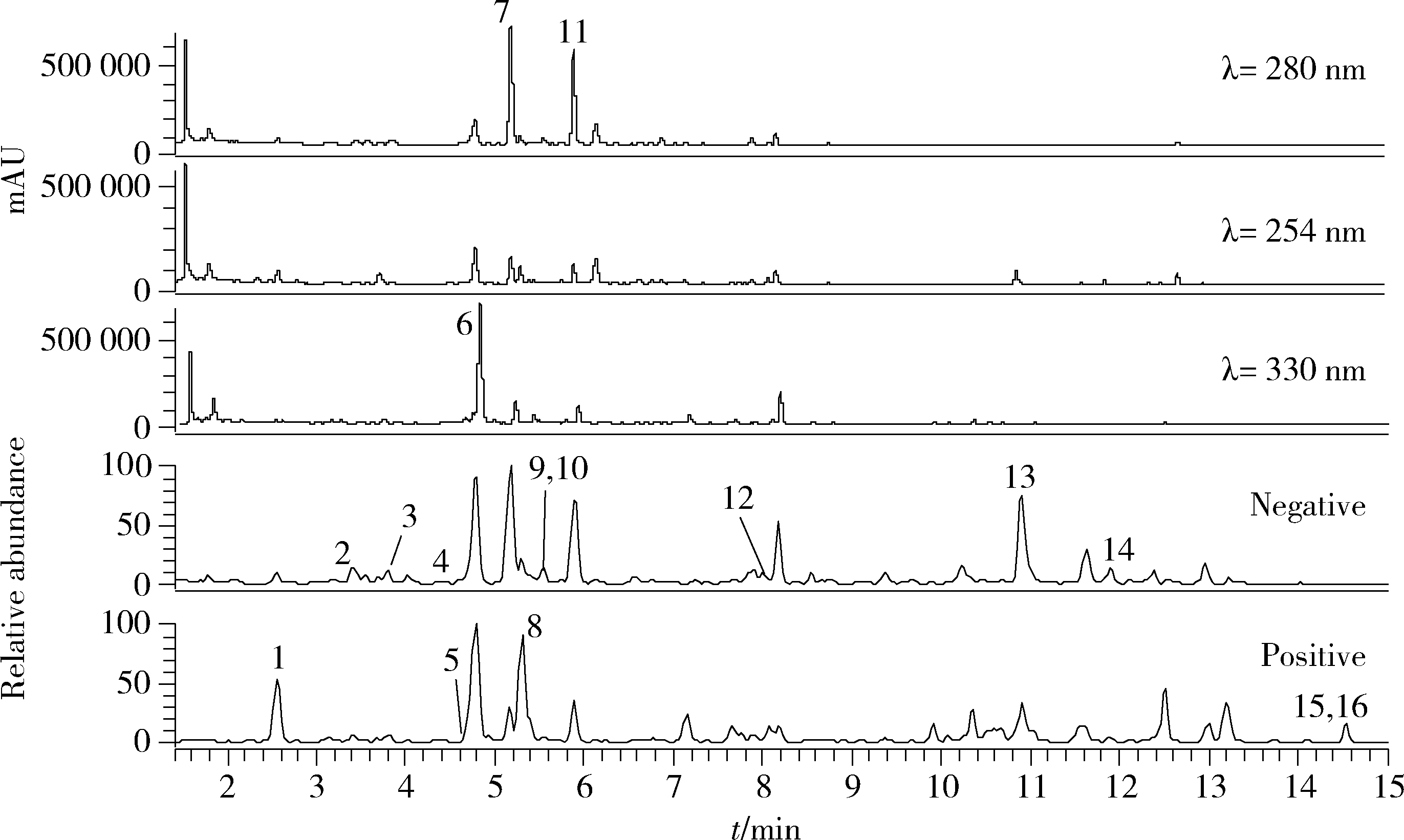
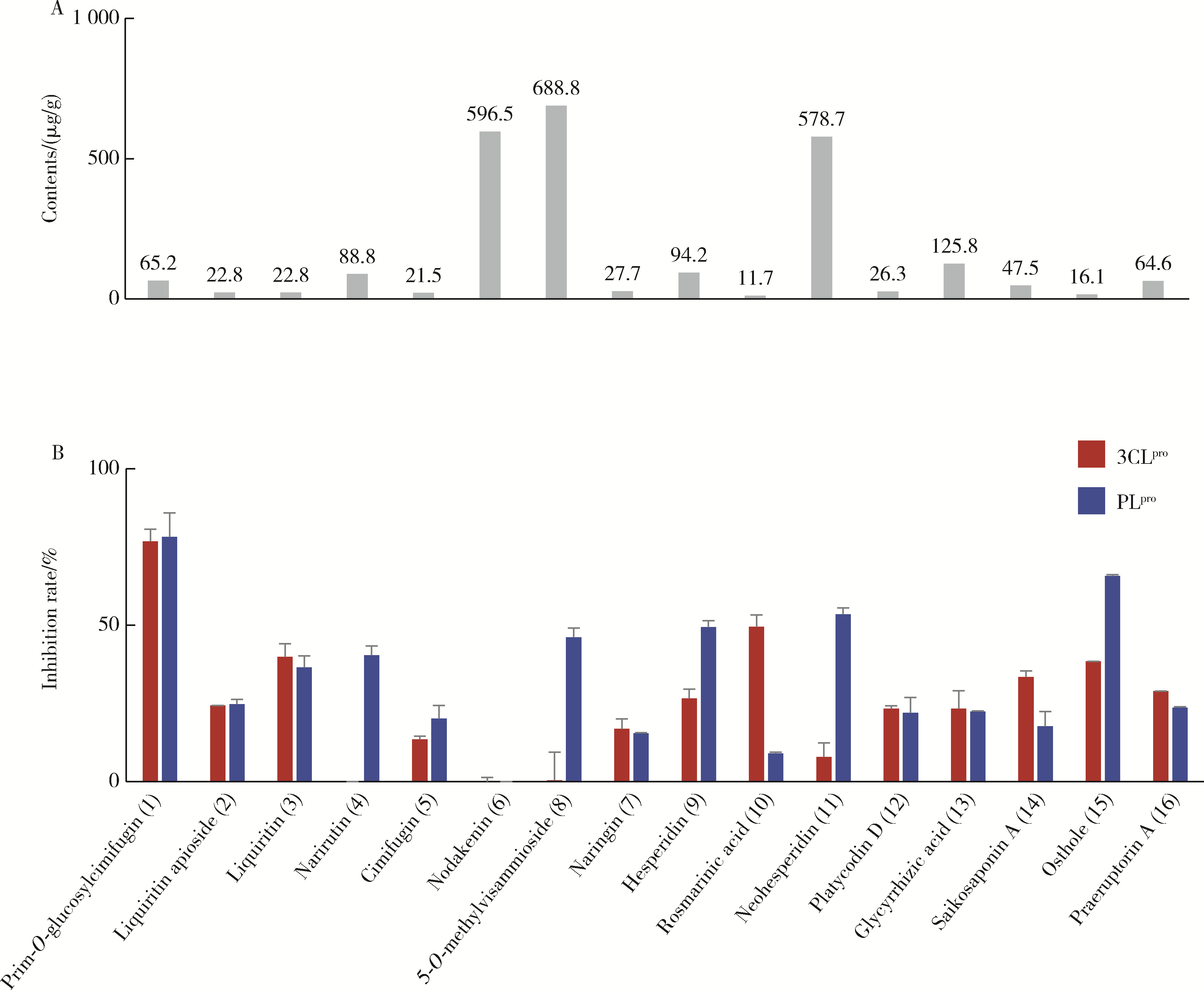
| 16 | 刘雯, 李峰, 孙春亮, 等. HPLC同时测定荆防颗粒中6种成分[J]. 中国实验方剂学杂志, 2016, 22 (17): 55- 58. |
| 17 | 冯雪, 高玉乔, 范琼瑛, 等. 采用LC-ESI/MS方法同时测定荆防败毒口服液中4个有效成分含量[J]. 药物分析杂志, 2017, 37 (8): 1489- 1496. |
| 18 | 梁红宝, 姜宇, 袁晓梅, 等. 基于GC-MS和UPLC-Q-Exactive MS技术的荆防颗粒化学成分研究[J]. 中草药, 2022, 53 (6): 1697- 1708. |
| 19 |
Yi Y , Li J , Lai X , et al. Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection[J]. J Adv Res, 2022, 36, 201- 210.
doi: 10.1016/j.jare.2021.11.012 |
| 20 |
Shang Z , Xu L , Kuang Y , et al. Simultaneous determination of 35 constituents and elucidation of effective constituents in a multi-herb Chinese medicine formula Xiaoer-Feire-Kechuan[J]. J Pharm Anal, 2021, 11 (6): 717- 725.
doi: 10.1016/j.jpha.2021.01.003 |
| 21 |
Kuang Y , Li B , Fan J , et al. Antitussive and expectorant activities of licorice and its major compounds[J]. Bioorg Med Chem, 2018, 26 (1): 278- 284.
doi: 10.1016/j.bmc.2017.11.046 |
| 22 |
Shang ZP , Xu LL , Xiao Y , et al. A global profiling strategy using comprehensive two-dimensional liquid chromatography coupled with dual-mass spectrometry platforms: Chemical analysis of a multi-herb Chinese medicine formula as a case study[J]. J Chromatogr A, 2021, 1642, 462021.
doi: 10.1016/j.chroma.2021.462021 |
| 23 |
Song W , Qiao X , Chen K , et al. Biosynthesis-based quantitative analysis of 151 secondary metabolites of licorice to differentiate medicinal Glycyrrhiza species and their hybrids[J]. Anal Chem, 2017, 89 (5): 3146- 3153.
doi: 10.1021/acs.analchem.6b04919 |
| 24 |
Wang S , Qian Y , Sun M , et al. Holistic quality evaluation of Saposhnikoviae Radix (Saposhnikovia divaricata) by reversed-phase ultra-high performance liquid chromatography and hydrophi-lic interaction chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry-based untargeted metabolomics[J]. Arab J Chem, 2020, 13 (12): 8835- 8847.
doi: 10.1016/j.arabjc.2020.10.013 |
| 25 | Bai Y , Zheng Y , Pang W , et al. Identification and comparison of constituents of Aurantii Fructus and Aurantii Fructus Immaturus by UFLC-DAD-Triple TOF-MS/MS[J]. Molecules, 2018, 23 (4): 1- 15. |
| 26 |
Chu S , Chen L , Xie H , et al. Comparative analysis and chemical profiling of different forms of Peucedani Radix[J]. J Pharmaceut Biomed Anal, 2020, 189, 113410.
doi: 10.1016/j.jpba.2020.113410 |
| 27 |
Wan M , Zhang Y , Yang Y , et al. Analysis of the chemical composition of Angelicae Pubescentis Radix by ultra-performance liquid chromatography and quadrupole time-of-flight tandem mass spectrometry[J]. J Chin Pharm Sci, 2019, 28 (3): 145- 159.
doi: 10.5246/JCPS.2019.03.014 |
| 28 |
Huang W , Zhou H , Yuan M , et al. Comprehensive characterization of the chemical constituents in Platycodon grandiflorum by an integrated liquid chromatography-mass spectrometry strategy[J]. J Chromatogr A, 2021, 1654, 462477.
doi: 10.1016/j.chroma.2021.462477 |
| 29 | 郭敏娜, 刘素香, 赵艳敏, 等. 基于HPLC-Q-TOF-MS技术的柴胡化学成分分析[J]. 中草药, 2016, 12 (47): 2044- 2052. |
| 30 | 郑单单, 魏文峰, 霍金海, 等. 基于UPLC-Q-TOF-MS技术的芪风固表颗粒血清药物化学研究[J]. 中草药, 2021, 52 (3): 643- 652. |
| 31 | 魏飞亭, 程昊, 乔日发, 等. UPLC-Q-TOF/MS鉴定大鼠灌服枳壳提取物后的入血成分及其代谢产物[J]. 中国实验方剂学杂志, 2020, 26 (21): 161- 172. |
|
||