Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (5): 981-990. doi: 10.19723/j.issn.1671-167X.2022.05.027
Previous Articles Next Articles
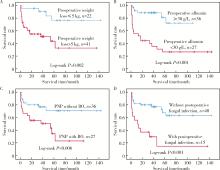
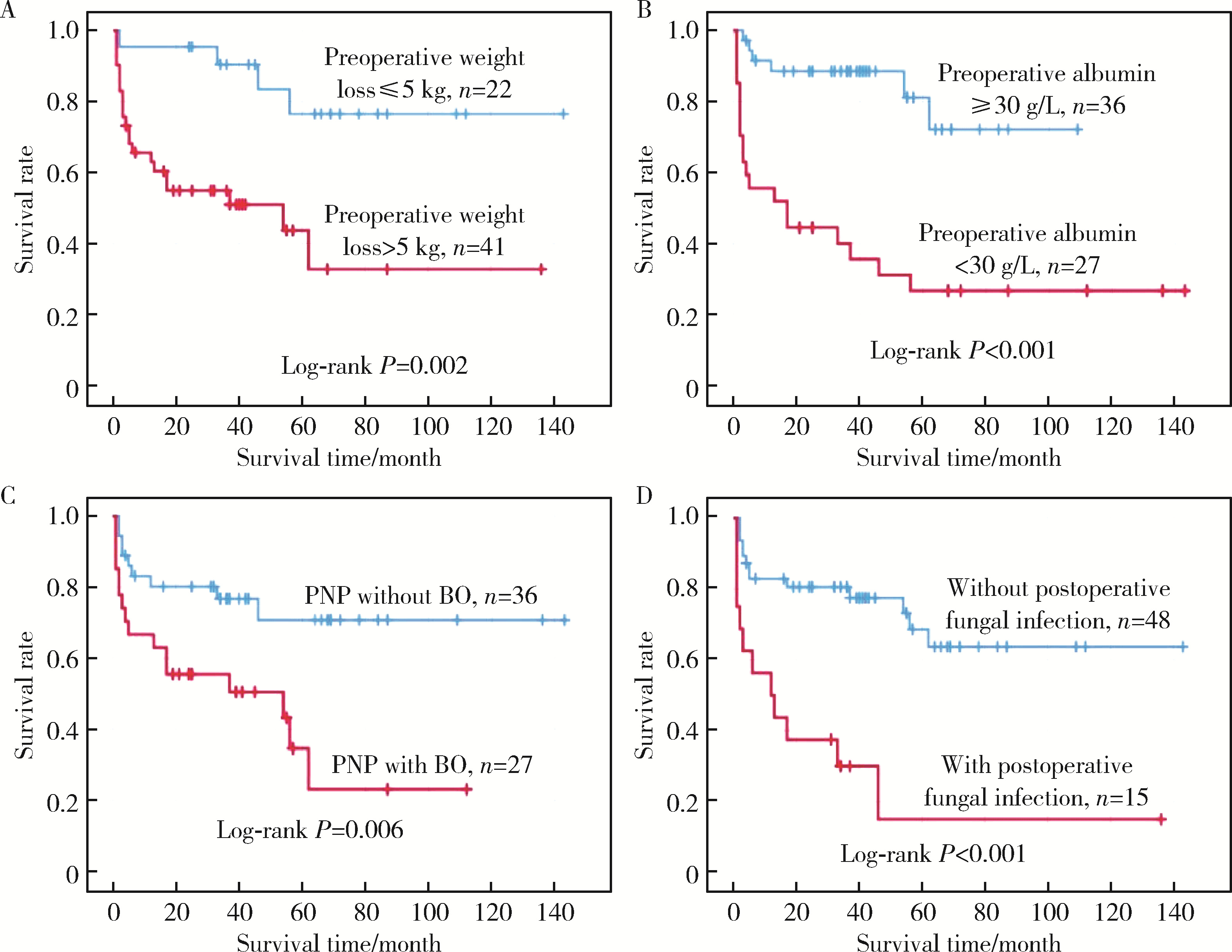
Factors associated with long-term survival in critically ill patients following surgery for solid tumors complicated with paraneoplastic pemphigus
Jia-xin PAN1,Sai-nan ZHU2,Shuang-ling LI1,*( ),Dong-xin WANG1,3
),Dong-xin WANG1,3
- 1. Department of Critical Care Medicine, Peking University First Hospital, Beijing 100034, China
2. Department of Biostatistics, Peking University First Hospital, Beijing 100034, China
3. Department of Anesthesiology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R593.2
| 1 |
Anhalt GJ , Kim SC , Stanley JR , et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia[J]. N Engl J Med, 1990, 323 (25): 1729- 1735.
doi: 10.1056/NEJM199012203232503 |
| 2 | Nguyen VT , Ndoye A , Bassler KD , et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus[J]. Arch Dermatol, 2001, 137 (2): 193- 206. |
| 3 |
Paolino G , Didona D , Magliulo G , et al. Paraneoplastic pemphigus: insight into the autoimmune pathogenesis, clinical features and therapy[J]. Int J Mol Sci, 2017, 18 (12): 2532.
doi: 10.3390/ijms18122532 |
| 4 |
Solimani F , Maglie R , Pollmann R , et al. Thymoma-associated paraneoplastic autoimmune multiorgan syndrome: from pemphigus to lichenoid dermatitis[J]. Front Immunol, 2019, 10, 1413.
doi: 10.3389/fimmu.2019.01413 |
| 5 |
Jelti L , Cordel N , Gillibert A , et al. Incidence and mortality of pemphigus in France[J]. J Invest Dermatol, 2019, 139 (2): 469- 473.
doi: 10.1016/j.jid.2018.07.042 |
| 6 |
Cao L , Wang F , Du XY , et al. Chronic lymphocytic leukemia-associated paraneoplastic pemphigus: potential cause and therapeutic strategies[J]. Sci Rep, 2020, 10 (1): 16357.
doi: 10.1038/s41598-020-73131-y |
| 7 |
Tirado-Sanchez A , Bonifaz A . Paraneoplastic pemphigus. A life-threatening autoimmune blistering disease[J]. Actas Dermosifi-liogr, 2017, 108 (10): 902- 910.
doi: 10.1016/j.ad.2017.04.024 |
| 8 |
Kishi T , Nakata J , Yamada T , et al. Fatal progression of bron-chiolitis obliterans in spite of complete remission of follicular lymphoma and paraneoplastic pemphigus[J]. Ann Hematol, 2022, 101 (2): 453- 455.
doi: 10.1007/s00277-021-04499-8 |
| 9 |
Tsuchisaka A , Numata S , Teye K , et al. Epiplakin is a paraneoplastic pemphigus autoantigen and related to bronchiolitis obliterans in Japanese patients[J]. J Invest Dermatol, 2016, 136 (2): 399- 408.
doi: 10.1038/JID.2015.408 |
| 10 |
Aguilar PR , Michelson AP , Isakow W . Obliterative bronchiolitis[J]. Transplantation, 2016, 100 (2): 272- 283.
doi: 10.1097/TP.0000000000000892 |
| 11 |
Krain RL , Kushner CJ , Tarazi M , et al. Assessing the correlation between disease severity indices and quality of life measurement tools in pemphigus[J]. Front Immunol, 2019, 10, 2571.
doi: 10.3389/fimmu.2019.02571 |
| 12 |
Czernik A , Camilleri M , Pittelkow MR , et al. Paraneoplastic autoimmune multiorgan syndrome: 20 years after[J]. Int J Dermatol, 2011, 50 (8): 905- 914.
doi: 10.1111/j.1365-4632.2011.04868.x |
| 13 |
Yong AA , Tey HL . Paraneoplastic pemphigus[J]. Australas J Dermatol, 2013, 54 (4): 241- 250.
doi: 10.1111/j.1440-0960.2012.00921.x |
| 14 | Wang J , Zhu X , Li R , et al. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China[J]. Arch Dermatol, 2005, 141 (10): 1285- 1293. |
| 15 |
Leger S , Picard D , Ingen-Housz-Oro S , et al. Prognostic factors of paraneoplastic pemphigus[J]. Arch Dermatol, 2012, 148 (10): 1165.
doi: 10.1001/archdermatol.2012.1830 |
| 16 |
Dong Y , Wang M , Nong L , et al. Clinical and laboratory characterization of 114 cases of Castleman disease patients from a single centre: paraneoplastic pemphigus is an unfavourable prognostic factor[J]. Br J Haematol, 2015, 169 (6): 834- 842.
doi: 10.1111/bjh.13378 |
| 17 |
Ouedraogo E , Gottlieb J , de Masson A , et al. Risk factors for death and survival in paraneoplastic pemphigus associated with hematologic malignancies in adults[J]. J Am Acad Dermatol, 2019, 80 (6): 1544- 1549.
doi: 10.1016/j.jaad.2018.03.043 |
| 18 | Wang M , Li F , Wang X , et al. Features and risk factors for paraneoplastic autoimmune multiorgan syndrome in 145 Chinese patients[J]. Acta Derm Venereol, 2020, 100 (18): adv00312. |
| 19 | Kartan S , Shi VY , Clark AK , et al. Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment[J]. Am J Clin Dermatol, 2017, 18 (1): 105- 126. |
| [1] | ZHOU Guang-ping,ZHOU Qian-yun,ZHU Ji-hong. A case report of TAFRO syndrome [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 814-817. |
|
||