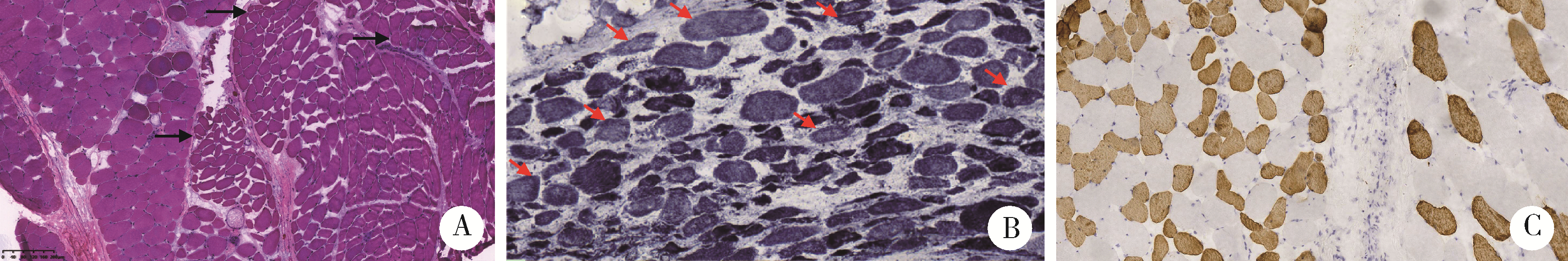
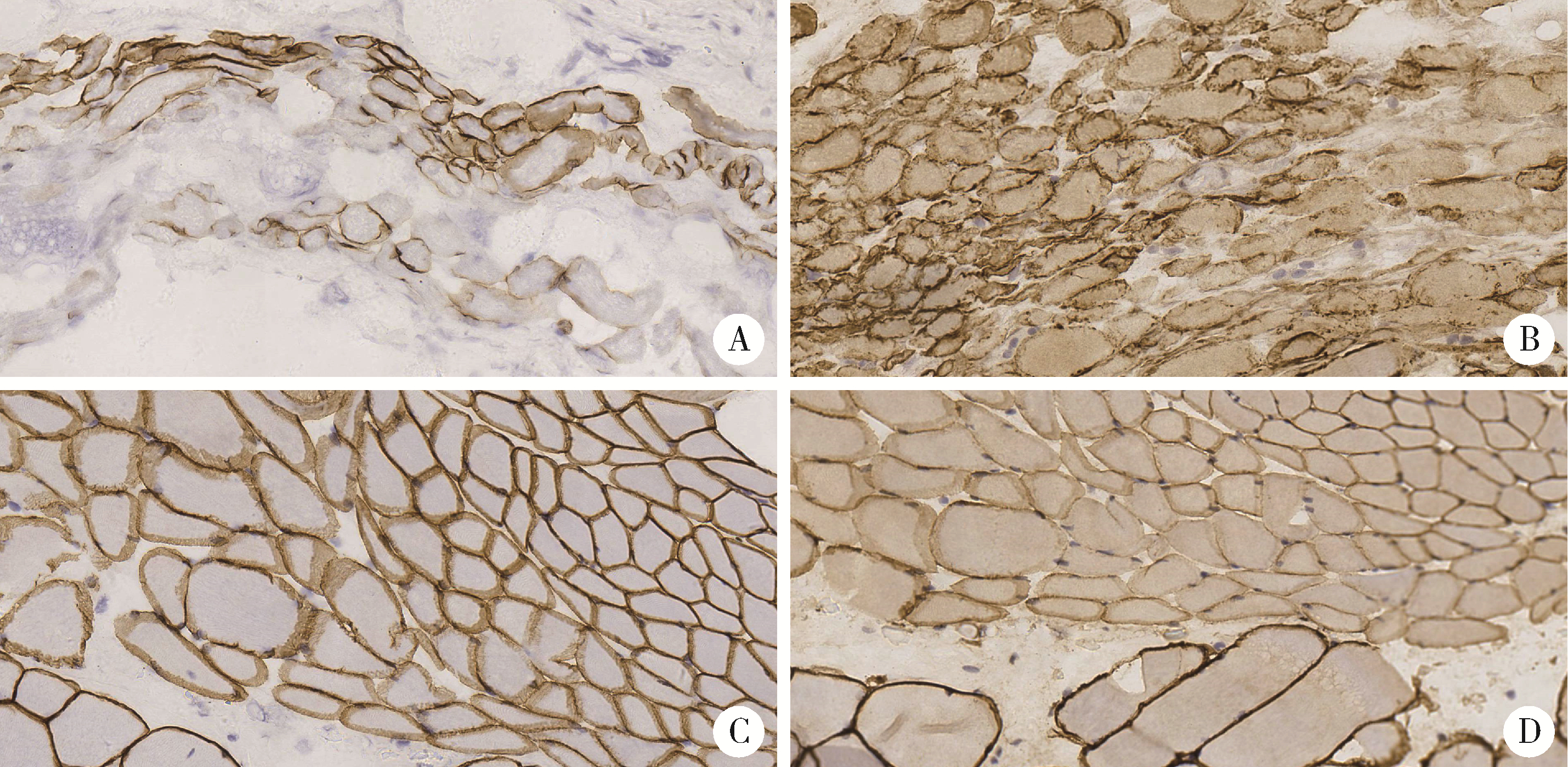
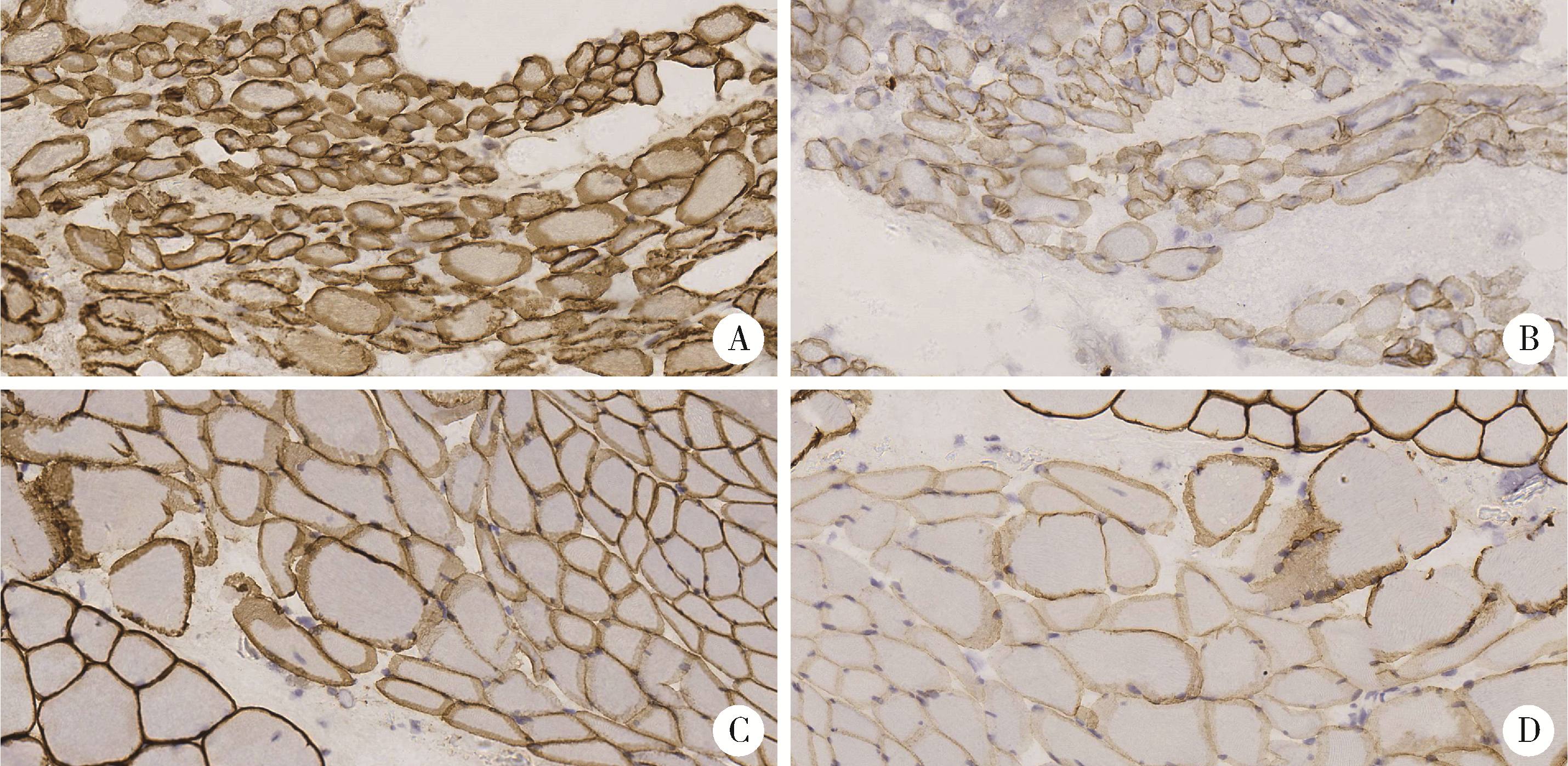
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 283-291. doi: 10.19723/j.issn.1671-167X.2023.02.012
Previous Articles Next Articles
Pathologic features of paraspinal muscle biopsies in patients with adolescent idiopathic scoliosis
Dan-feng ZHENG1,Jun-yu LI2,3,Jia-xi LI4,Ying-shuang ZHANG5,Yan-feng ZHONG1,Miao YU2,3,*( )
)
- 1. Department of Pathology, School of Basic Medical Sciences Peking University/Peking University Third Hospital, Beijing 100191, China
2. Departmant of Orthopaedics, Peking University Third Hospital, Beijing 100191, China
3. Beijing Key Laboratory of Spinal Disease Research, Peking University Third Hospital, Beijing 100191, China
4. School of Basic Medical Sciences, Peking University, Beijing 100191, China
5. Departmant of Neurology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R682.3
| 1 |
Cheng JC , Castelein RM , Chu WC , et al. Adolescent idiopathic scoliosis[J]. Nat Rev Dis Primers, 2015, 1, 15030.
doi: 10.1038/nrdp.2015.30 |
| 2 | Weinstein SL . The natural history of adolescent idiopathic scoliosis[J]. J Pediatr Orthop, 2019, 39 (6): S44- S46. |
| 3 |
Spencer GS , Eccles MJ . Spinal muscle in scoliosis: Part 2. The proportion and size of type 1 and type 2 skeletal muscle fibres measured using a computer-controlled microscope[J]. J Neurol Sci, 1976, 30 (1): 143- 154.
doi: 10.1016/0022-510X(76)90262-8 |
| 4 | Fidler MW , Jowett RL . Muscle imbalance in the aetiology of sco-liosis[J]. J Bone Joint Surg B, 1976, 58 (2): 200- 201. |
| 5 |
Khosla S , Tredwell SJ , Day B , et al. An ultrastructural study of multifidus muscle in progressive idiopathic scoliosis. Changes resulting from a sarcolemmal defect at the myotendinous junction[J]. J Neurol Sci, 1980, 46 (1): 13- 31.
doi: 10.1016/0022-510X(80)90040-4 |
| 6 |
Tidball JG . Myotendinous junction: Morphological changes and mechanical failure associated with muscle cell atrophy[J]. Exp Mol Pathol, 1984, 40 (1): 1- 12.
doi: 10.1016/0014-4800(84)90060-1 |
| 7 |
Wajchenberg M , Martins DE , Luciano RP , et al. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: A cross-sectional study[J]. Medicine (Baltimore), 2015, 94 (8): e598.
doi: 10.1097/MD.0000000000000598 |
| 8 |
Luciano Rde P , Puertas EB , Martins DE , et al. Adolescent idiopathic scoliosis without limb weakness: A differential diagnosis of core myopathy?[J]. BMC Musculoskelet Disord, 2015, 16, 179.
doi: 10.1186/s12891-015-0629-8 |
| 9 |
Zoabli G , Mathieu PA , Aubin CE . Magnetic resonance imaging of the erector spinae muscles in Duchenne muscular dystrophy: Implication for scoliotic deformities[J]. Scoliosis, 2008, 3, 21.
doi: 10.1186/1748-7161-3-21 |
| 10 |
Nash CL Jr , Moe JH . A study of vertebral rotation[J]. J Bone Joint Surg Am, 1969, 51 (2): 223- 229.
doi: 10.2106/00004623-196951020-00002 |
| 11 | Dubowitz V , Sewry CA , Oldfors A , et al. Muscle biopsy: A practical approach[M]. 4th ed. Oxford, England: Saunders Else-vier, 2013. |
| 12 |
Altaf F , Gibson A , Dannawi Z , et al. Adolescent idiopathic sco-liosis[J]. BMJ, 2013, 346, f2508.
doi: 10.1136/bmj.f2508 |
| 13 |
Brzoska E , Kalkowski L , Kowalski K , et al. Muscular contribution to adolescent idiopathic scoliosis from the perspective of stem cell-based regenerative medicine[J]. Stem Cells Dev, 2019, 28 (16): 1059- 1077.
doi: 10.1089/scd.2019.0073 |
| 14 |
Veldhuizen AG , Wever DJ , Webb PJ . The aetiology of idiopathic scoliosis: Biomechanical and neuromuscular factors[J]. Eur Spine J, 2000, 9 (3): 178- 184.
doi: 10.1007/s005860000142 |
| 15 |
Meier MP , Klein MP , Krebs D , et al. Fiber transformations in multifidus muscle of young patients with idiopathic scoliosis[J]. Spine (Phila Pa 1976), 1997, 22 (20): 2357- 2364.
doi: 10.1097/00007632-199710150-00008 |
| 16 | Bylund P , Jansson E , Dahlberg E , et al. Muscle fiber types in thoracic erector spinae muscles[J]. Clin Orthop Relat Res, 1987, (214): 222- 228. |
| 17 |
Mannion AF , Meier M , Grob D , et al. Paraspinal muscle fibre type alterations associated with scoliosis: an old problem revisited with new evidence[J]. Eur Spine J, 1998, 7 (4): 289- 293.
doi: 10.1007/s005860050077 |
| 18 |
Hoffman EP , Brown RH Jr , Kunkel LM . Dystrophin: The protein product of the Duchenne muscular dystrophy locus[J]. Cell, 1987, 51 (6): 919- 928.
doi: 10.1016/0092-8674(87)90579-4 |
| 19 |
Ervasti JM , Campbell KP . Membrane organization of the dystrophin-glycoprotein complex[J]. Cell, 1991, 66 (6): 1121- 1231.
doi: 10.1016/0092-8674(91)90035-W |
| 20 |
Houang EM , Sham YY , Bates FS , et al. Muscle membrane integrity in Duchenne muscular dystrophy: Recent advances in copolymer-based muscle membrane stabilizers[J]. Skelet Muscle, 2018, 8 (1): 31.
doi: 10.1186/s13395-018-0177-7 |
| 21 |
Yiu EM , Kornberg AJ . Duchenne muscular dystrophy[J]. J Paediatr Child Health, 2015, 51 (8): 759- 764.
doi: 10.1111/jpc.12868 |
| 22 |
Wilson K , Faelan C , Patterson-Kane JC , et al. Duchenne and Becker muscular dystrophies: A review of animal models, clinical end points, and biomarker quantification[J]. Toxicol Pathol, 2017, 45 (7): 961- 976.
doi: 10.1177/0192623317734823 |
| 23 | Goebel HH , Sewry CA , Weller RO . Muscle disease: Pathology and genetics[M]. 2nd ed. New Jersey, USA: Wiley-Blackwell, 2013: 95- 100. |
| 24 | Gherardi RK . Pathogenic aspects of dermatomyositis, polymyositis and overlap myositis[J]. Presse Med, 2011, 40 (4 Pt 2): e209- e218. |
| 25 |
Samaan MC , Missiuna P , Peterson D , et al. Understanding the role of the immune system in adolescent idiopathic scoliosis: Immunometabolic CONnections to Scoliosis (ICONS) study protocol[J]. BMJ Open, 2016, 6 (7): e011812.
doi: 10.1136/bmjopen-2016-011812 |
| 26 |
Rudrapatna S , Peterson D , Missiuna P , et al. Understanding muscle-immune interactions in adolescent idiopathic scoliosis: A feasibility study[J]. Pilot Feasibility Stud, 2017, 3, 50.
doi: 10.1186/s40814-017-0193-0 |
| 27 |
Van Gennip JLM , Boswell CW , Ciruna B . Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis[J]. Sci Adv, 2018, 4 (12): eaav1781.
doi: 10.1126/sciadv.aav1781 |
| 28 |
Dalkilic I , Kunkel LM . Muscular dystrophies: Genes to pathoge-nesis[J]. Curr Opin Genet Dev, 2003, 13 (3): 231- 238.
doi: 10.1016/S0959-437X(03)00048-0 |
| 29 |
Fiorillo AA , Heier CR , Novak JS , et al. TNF-α-induced micro-RNAs control dystrophin expression in Becker muscular dystrophy[J]. Cell Rep, 2015, 12 (10): 1678- 1690.
doi: 10.1016/j.celrep.2015.07.066 |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Yuxuan TIAN,Mingjian RUAN,Yi LIU,Derun LI,Jingyun WU,Qi SHEN,Yu FAN,Jie JIN. Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 567-574. |
| [3] | Kaifeng YAO,Mingjian RUAN,Derun LI,Yuxuan TIAN,Yuke CHEN,Yu FAN,Yi LIU. Diagnostic efficacy of targeted biopsy combined with regional systematic biopsy in prostate cancer in patients with PI-RADS 4-5 [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 575-581. |
| [4] | Hejun SHEN,Chongyan SHI,Qing ZHENG,Yu HUANG,Tao JING. Investigation on the current situation and influencing factors of sitting time and health literacy among high school students in China [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 239-246. |
| [5] | Yi LIU,Chang-wei YUAN,Jing-yun WU,Qi SHEN,Jiang-xi XIAO,Zheng ZHAO,Xiao-ying WANG,Xue-song LI,Zhi-song HE,Li-qun ZHOU. Diagnostic efficacy of prostate cancer using targeted biopsy with 6-core systematic biopsy for patients with PI-RADS 5 [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 812-817. |
| [6] | Chang-wei YUAN,De-run LI,Zhi-hua LI,Yi LIU,Gang-zhi SHAN,Xue-song LI,Li-qun ZHOU. Application of dynamic contrast enhanced status in multiparametric magnetic resonance imaging for prostatic cancer with PI-RADS 4 lesion [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 838-842. |
| [7] | Meng-jie CUI,Qi MA,Man-man CHEN,Tao MA,Xin-xin WANG,Jie-yu LIU,Yi ZHANG,Li CHEN,Jia-nuo JIANG,Wen YUAN,Tong-jun GUO,Yan-hui DONG,Jun MA,Yi XING. Association between different growth patterns and metabolic syndrome in children and adolescents aged 7 to 17 years [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 415-420. |
| [8] | Jia-jia DANG,Shan CAI,Pan-liang ZHONG,Ya-qi WANG,Yun-fei LIU,Di SHI,Zi-yue CHEN,Yi-hang ZHANG,Pei-jin HU,Jing LI,Jun MA,Yi SONG. Association of outdoor artificial light at night exposure with overweight and obesity among children and adolescents aged 9 to 18 years in China [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 421-428. |
| [9] | Yan XIONG,Xin LI,Li LIANG,Dong LI,Li-min YAN,Xue-ying LI,Ji-ting DI,Ting LI. Evaluation of accuracy of pathological diagnosis based on thyroid core needle biopsy [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 234-242. |
| [10] | Yun-fei LIU,Jia-jia DANG,Pan-liang ZHONG,Ning MA,Di SHI,Yi SONG. Injury mortality among Chinese aged 5 to 24 years from 1990 to 2019 [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 498-504. |
| [11] | ZHOU Guang-ping,ZHOU Qian-yun,ZHU Ji-hong. A case report of TAFRO syndrome [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 814-817. |
| [12] | CHEN Man-man,YANG Zhao-geng,SU Bin-bin,LI Yan-hui,GAO Di,MA Ying,MA Tao,DONG Yan-hui,MA Jun. Analysis on the law of height growth spurt in adolescence of children and adolescents in Zhongshan City [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 506-510. |
| [13] | ZHANG Lei,LI Guo-liang,DANG Zong-hui, ,A yong,WU Ling-jie,LIU Li-jun. Analysis of bleeding risk in percutaneous renal biopsy in Tibet [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 298-301. |
| [14] | YANG Xue,SUN Wei,WANG Zhe,JI Ai-ping,BAI Jie. Clinical analysis of children and adolescents emergency dental trauma cases [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 384-389. |
| [15] | ZHOU Jing,LIU Yi. Cone-beam CT evaluation of temporomandibular joint in skeletal class Ⅱ female adolescents with different vertical patterns [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 109-119. |
|
||