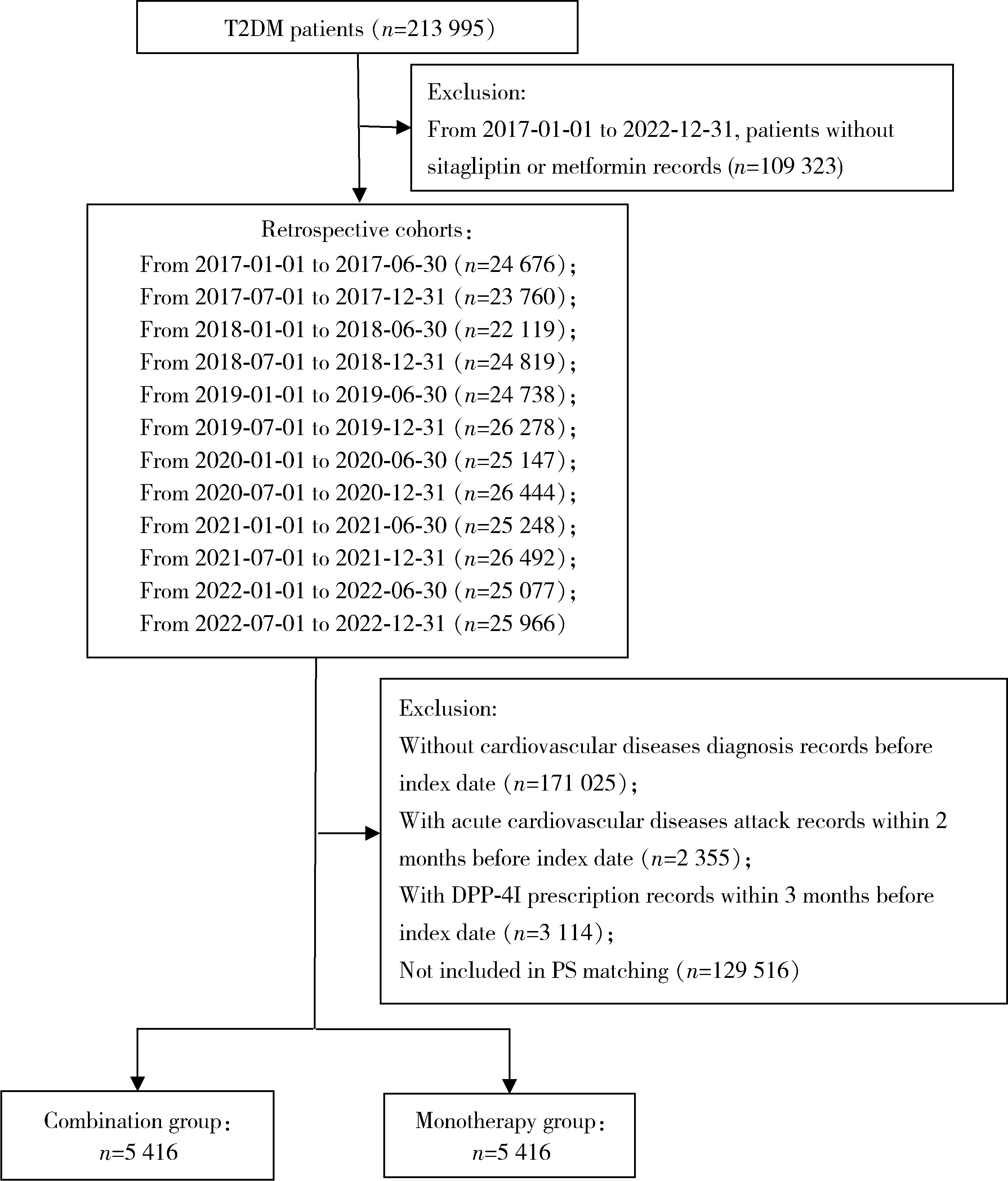
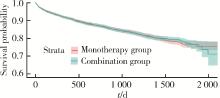
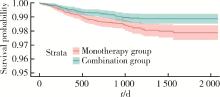
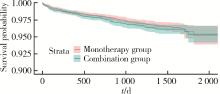
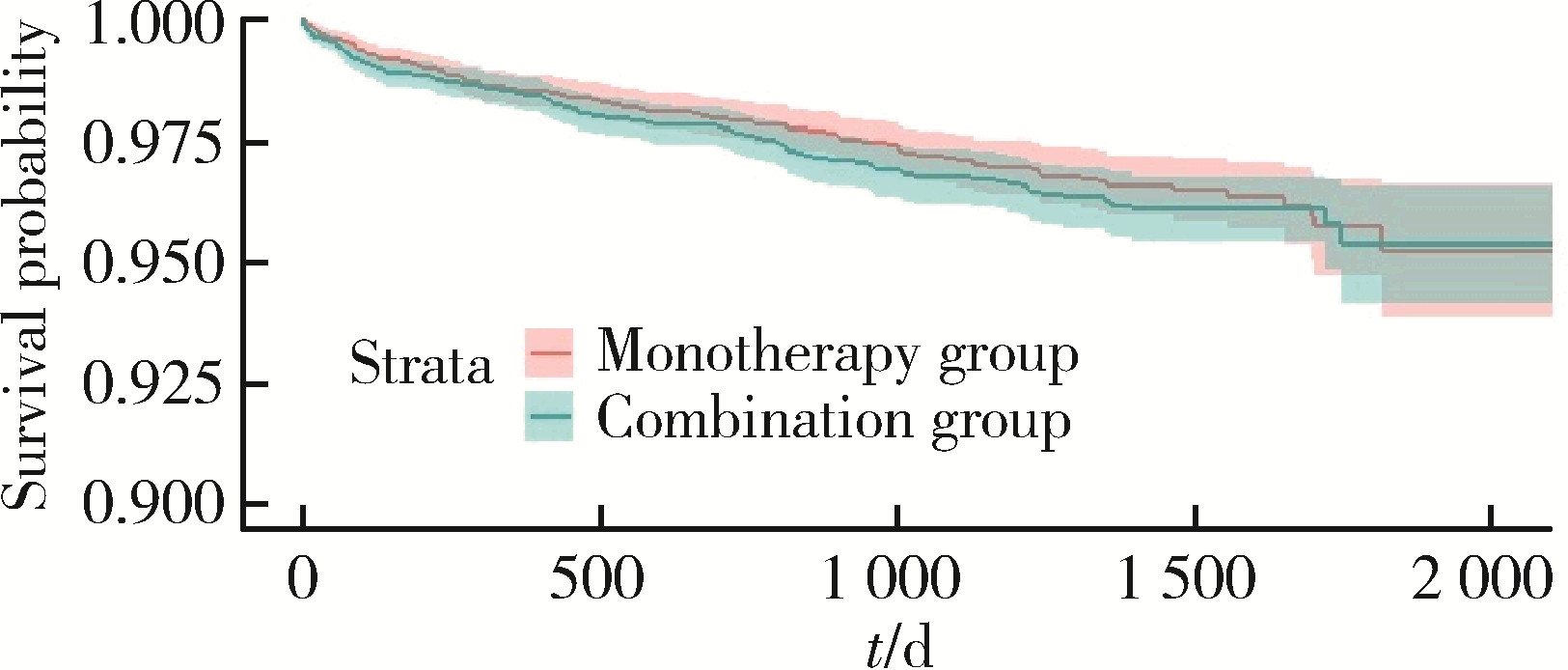
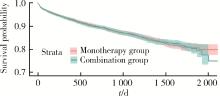
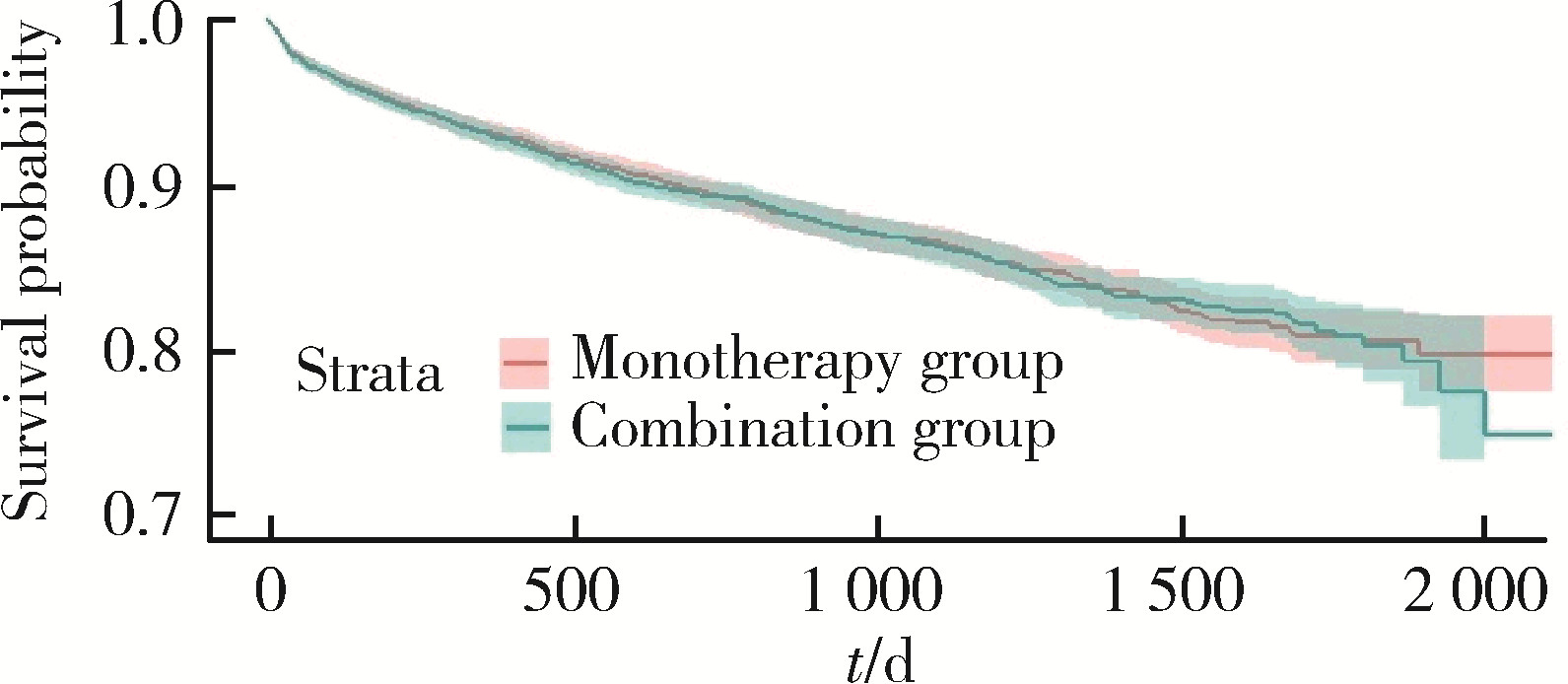
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (3): 424-430. doi: 10.19723/j.issn.1671-167X.2024.03.008
Previous Articles Next Articles
Cardiovascular safety of sitagliptin added to metformin in real world patients with type 2 diabetes
Zuoxiang LIU1,2,Xiaowei CHEN1,2,Houyu ZHAO1,2,Siyan ZHAN1,2,3,*( ),Feng SUN1,2,4,*(
),Feng SUN1,2,4,*( )
)
- 1. Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China
2. Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, Beijing 100191, China
3. Clinical Epidemiology Research Center, Peking University Third Hospital, Beijing 100191, China
4. Hainan Institute of Real World Data, Qionghai 571437, Hainan, China
CLC Number:
- R195.4
| 1 |
中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 国际内分泌代谢杂志, 2021, 41 (5): 482- 548.
doi: 10.3760/cma.j.cn121383-20210825-08063 |
| 2 |
Ji L , Hu D , Pan C , et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients[J]. Am J Med, 2013, 126 (10): 925.e11- 925.e 22.
doi: 10.1016/j.amjmed.2013.02.035 |
| 3 |
Schmidt AM . Diabetes mellitus and cardiovascular disease[J]. Arterioscler Thromb Vasc Biol, 2019, 39 (4): 558- 568.
doi: 10.1161/ATVBAHA.119.310961 |
| 4 |
Goldfine AB . Assessing the cardiovascular safety of diabetes therapies[J]. N Engl J Med, 2008, 359 (11): 1092- 1095.
doi: 10.1056/NEJMp0805758 |
| 5 |
Griffin SJ , Leaver JK , Irving GJ . Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes[J]. Diabetologia, 2017, 60 (9): 1620- 1629.
doi: 10.1007/s00125-017-4337-9 |
| 6 |
Garber AJ , Abrahamson MJ , Barzilay JI , et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive Type 2 diabetes management algorithm: 2020 executive summary[J]. Endocr Pract, 2020, 26 (1): 107- 139.
doi: 10.4158/CS-2019-0472 |
| 7 |
余学锋, 王爱红. 基层医疗机构二肽基肽酶4抑制剂临床应用常见问题专家指导建议[J]. 中国糖尿病杂志, 2022, 30 (2): 81- 85.
doi: 10.3969/j.issn.1006-6187.2022.02.001 |
| 8 |
Fei Y , Tsoi MF , Cheung BMY . Cardiovascular outcomes in trials of new antidiabetic drug classes: A network meta-analysis[J]. Cardiovasc Diabetol, 2019, 18 (1): 112.
doi: 10.1186/s12933-019-0916-z |
| 9 |
Green JB , Bethel MA , Armstrong PW , et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes[J]. N Engl J Med, 2015, 373 (3): 232- 242.
doi: 10.1056/NEJMoa1501352 |
| 10 |
El Sanadi CE , Ji X , Kattan MW . 3-point major cardiovascular event outcome for patients with T2D treated with dipeptidyl peptidase-4 inhibitor or glucagon-like peptide-1 receptor agonist in addition to metformin monotherapy[J]. Ann Transl Med, 2020, 8 (21): 1345.
doi: 10.21037/atm-20-4063 |
| 11 |
Zhao H , Chen X , Sun Y , et al. Associations between thiazolidi-nediones use and incidence of rheumatoid arthritis: A retrospective population-based cohort study[J]. Arthritis Care Res (Hoboken), 2024, 76 (4): 486- 496.
doi: 10.1002/acr.25277 |
| 12 |
Lin H , Tang X , Shen P , et al. Using big data to improve cardiovascular care and outcomes in China: A protocol for the Chinese electronic health records research in Yinzhou (CHERRY) study[J]. BMJ Open, 2018, 8 (2): e019698.
doi: 10.1136/bmjopen-2017-019698 |
| 13 |
Robins JM , Hernán MA , Rotnitzky A . Effect modification by time-varying covariates[J]. Am J Epidemiol, 2007, 166 (9): 994- 1002.
doi: 10.1093/aje/kwm231 |
| 14 |
Borah BJ , Moriarty JP , Crown WH , et al. Applications of propensity score methods in observational comparative effectiveness and safety research: Where have we come and where should we go?[J]. J Comp Eff Res, 2014, 3 (1): 63- 78.
doi: 10.2217/cer.13.89 |
| 15 |
中国医师协会内分泌代谢科医师分会. DPP-4抑制剂临床应用专家共识[J]. 中华内分泌代谢杂志, 2018, 34 (11): 899- 903.
doi: 10.3760/cma.j.issn.1000-6699.2018.11.001 |
| 16 |
Ussher JR , Drucker DJ . Cardiovascular biology of the incretin system[J]. Endocr Rev, 2012, 33 (2): 187- 215.
doi: 10.1210/er.2011-1052 |
| 17 |
Mccormick LM , Kydd AC , Read PA , et al. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease[J]. Circ Cardiovasc Imaging, 2014, 7 (2): 274- 281.
doi: 10.1161/CIRCIMAGING.113.000785 |
| 18 |
Anderluh M , Kocic G , Tomovic K , et al. Cross-talk between the dipeptidyl peptidase-4 and stromal cell-derived factor-1 in stem cell homing and myocardial repair: Potential impact of dipeptidyl peptidase-4 inhibitors[J]. Pharmacol Ther, 2016, 167, 100- 107.
doi: 10.1016/j.pharmthera.2016.07.009 |
| 19 |
Ussher JR , Drucker DJ . Cardiovascular actions of incretin-based therapies[J]. Circ Res, 2014, 114 (11): 1788- 1803.
doi: 10.1161/CIRCRESAHA.114.301958 |
| 20 |
Scirica BM , Bhatt DL , Braunwald E , et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus[J]. N Engl J Med, 2013, 369 (14): 1317- 1326.
doi: 10.1056/NEJMoa1307684 |
| 21 |
Zheng SL , Roddick AJ , Aghar-Jaffar R , et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: A systematic review and meta-analysis[J]. Jama, 2018, 319 (15): 1580- 1591.
doi: 10.1001/jama.2018.3024 |
| 22 |
Kaneko M , Narukawa M . Meta-analysis of dipeptidyl peptidase-4 inhibitors use and cardiovascular risk in patients with type 2 diabetes mellitus[J]. Diabetes Res Clin Pract, 2016, 116, 171- 182.
doi: 10.1016/j.diabres.2016.04.012 |
| 23 |
Giugliano D , Longo M , Signoriello S , et al. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: A network meta-analysis of 23 CVOTs[J]. Cardiovasc Diabetol, 2022, 21 (1): 42.
doi: 10.1186/s12933-022-01474-z |
| 24 |
Ou SM , Shih CJ , Chao PW , et al. Effects on clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus[J]. Ann Intern Med, 2015, 163 (9): 663- 672.
doi: 10.7326/M15-0308 |
| 25 |
Baksh S , Wen J , Mansour O , et al. Dipeptidyl peptidase-4 inhi-bitor cardiovascular safety in patients with type 2 diabetes, with cardiovascular and renal disease: A retrospective cohort study[J]. Sci Rep, 2021, 11 (1): 16637.
doi: 10.1038/s41598-021-95687-z |
| 26 |
Noguchi Y , Yoshizawa S , Tachi T , et al. Effect of dipeptidyl peptidase-4 inhibitors vs. metformin on major cardiovascular events using spontaneous reporting system and real-world database study[J]. J Clin Med, 2022, 11 (17): 4988.
doi: 10.3390/jcm11174988 |
| 27 |
Ray WA . Evaluating medication effects outside of clinical trials: New-user designs[J]. Am J Epidemiol, 2003, 158 (9): 915- 920.
doi: 10.1093/aje/kwg231 |
| [1] | Tianjiao HOU,Zhibo ZHOU,Zhuqing WANG,Mengying WANG,Siyue WANG,Hexiang PENG,Huangda GUO,Yixin LI,Hanyu ZHANG,Xueying QIN,Yiqun WU,Hongchen ZHENG,Jing LI,Tao WU,Hongping ZHU. Gene-gene/gene-environment interaction of transforming growth factor-β signaling pathway and the risk of non-syndromic oral clefts [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 384-389. |
| [2] | CHEN Ping,LI Ze-ming,GUO Yi,SUN Xin-ying,Edwin B. FISHER. To explore medication adherence of patients with type 2 diabetes mellitus using the latent profile analysis based on the Big Five personality theory [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 530-535. |
| [3] | Lu XU,Lu CHEN,Dong-sheng FAN,Jing-nan FENG,Li-li LIU,Si-yan ZHAN,Sheng-feng WANG. Calculation of the prevalence of progressive muscular atrophy among adults in China based on urban medical insurance data from 15 provinces [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 521-526. |
| [4] | YANG Yu, ZHAO Hou-yu, ZHAN Si-yan. Necessity and feasibility of data sharing of cohort studies#br# #br# [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 381-385. |
| [5] | REN Qiao-meng, WANG Li-min, PENG Dan-lu, GUO Yan. Influence of awareness on the behaviors of Chinese adults with diabetes mellitus [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 451-454. |
|
||