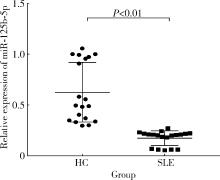
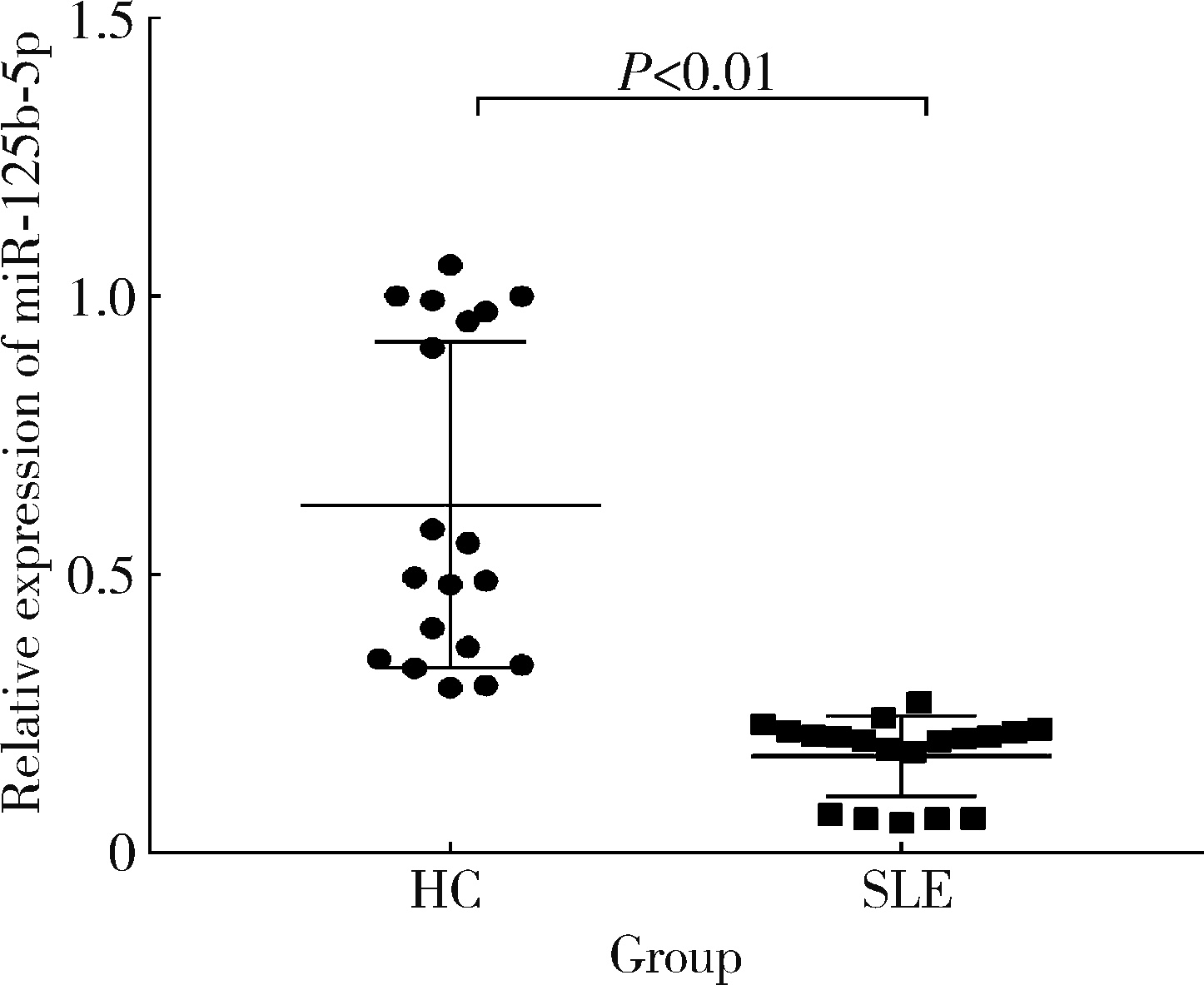
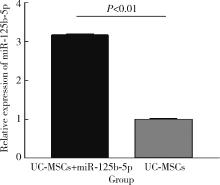
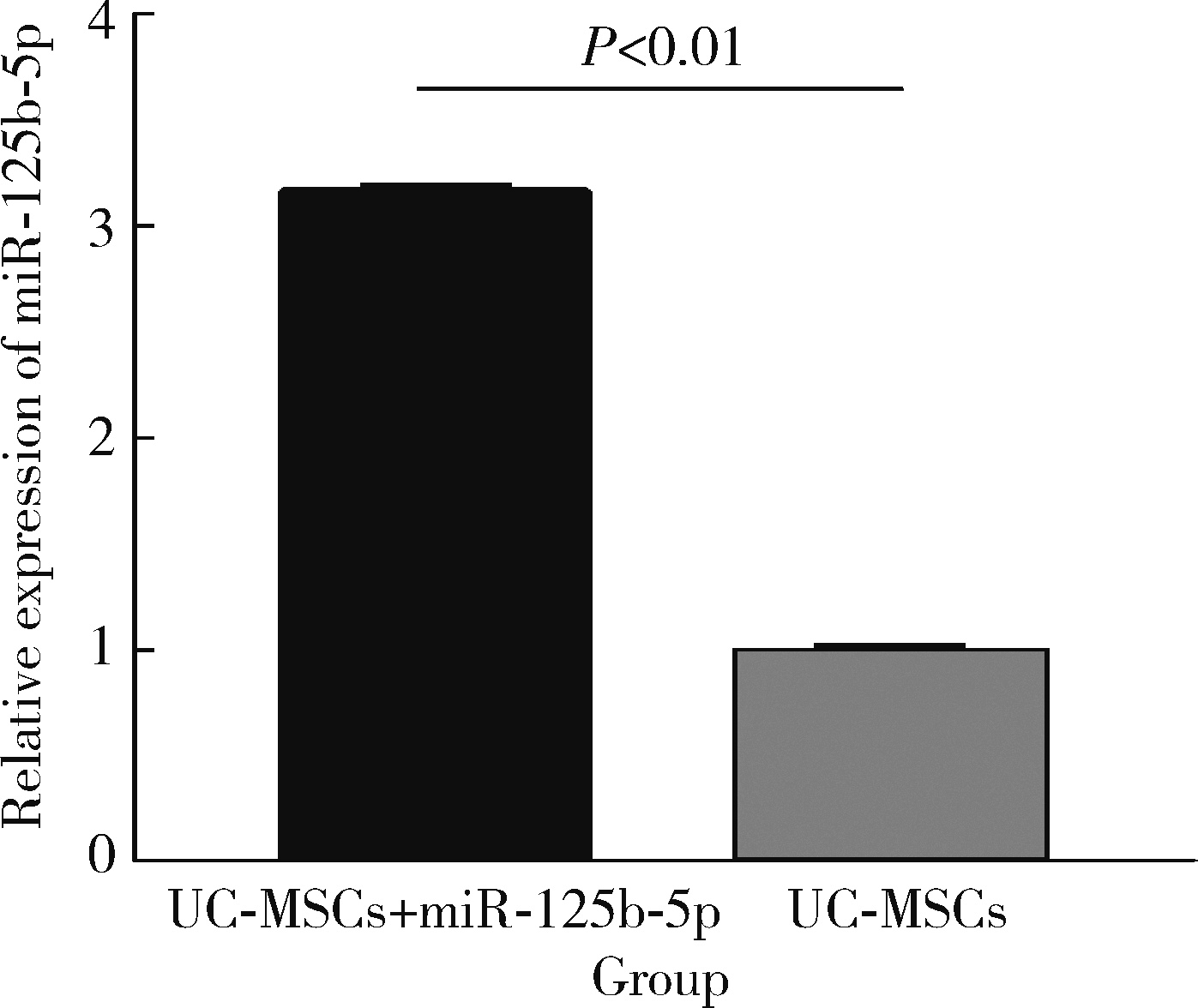
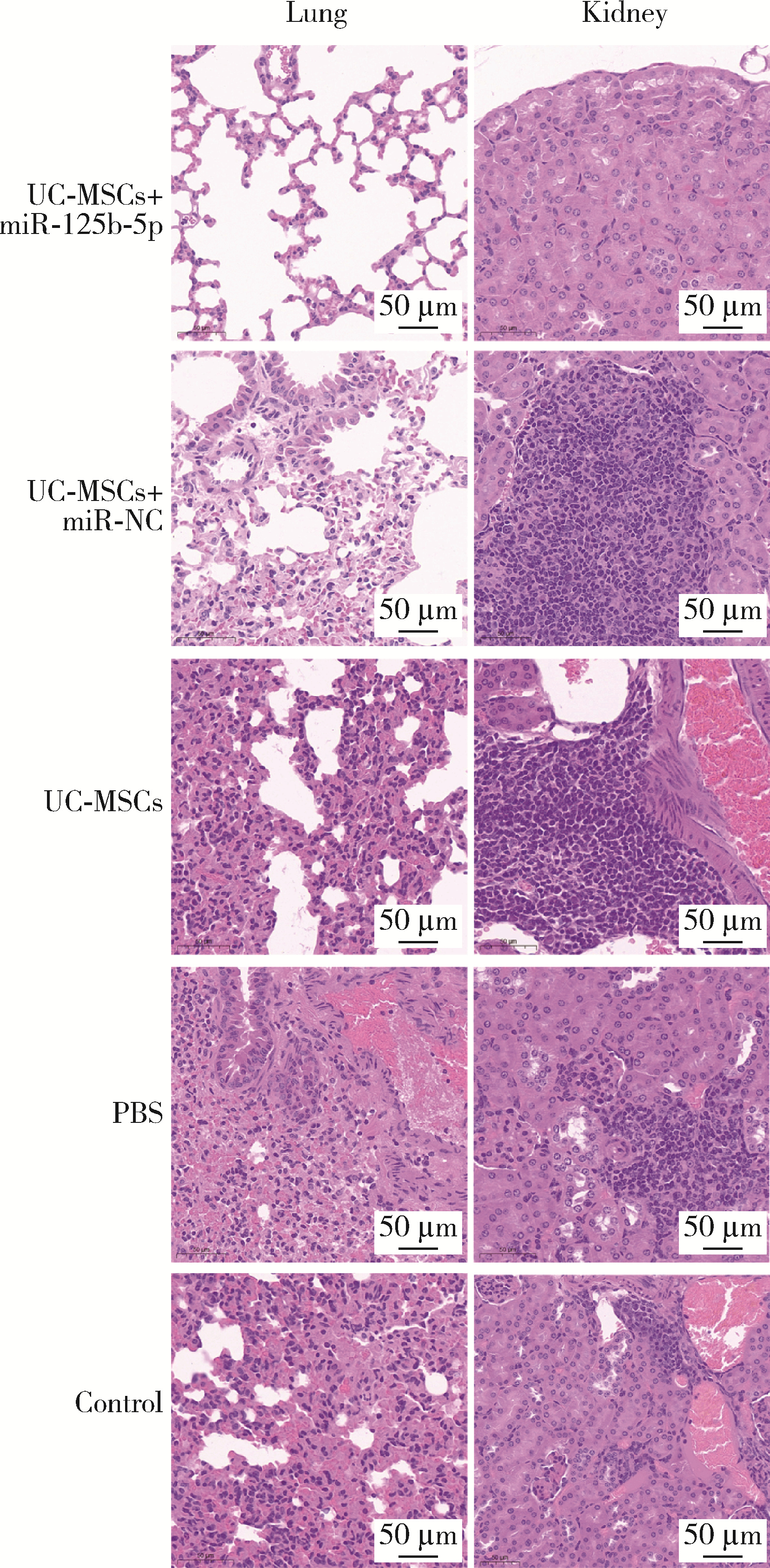
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 860-867. doi: 10.19723/j.issn.1671-167X.2024.05.017
Previous Articles Next Articles
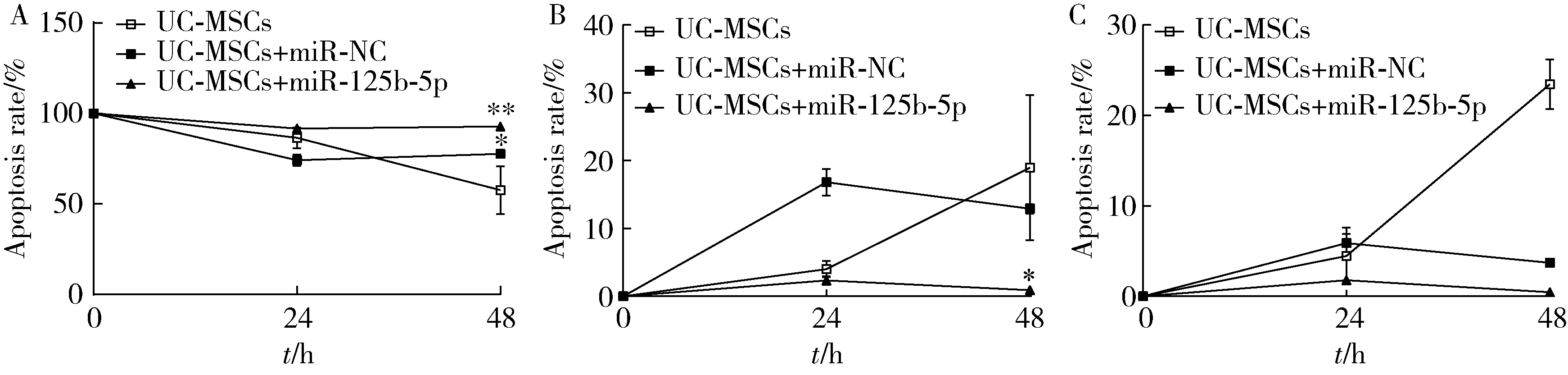
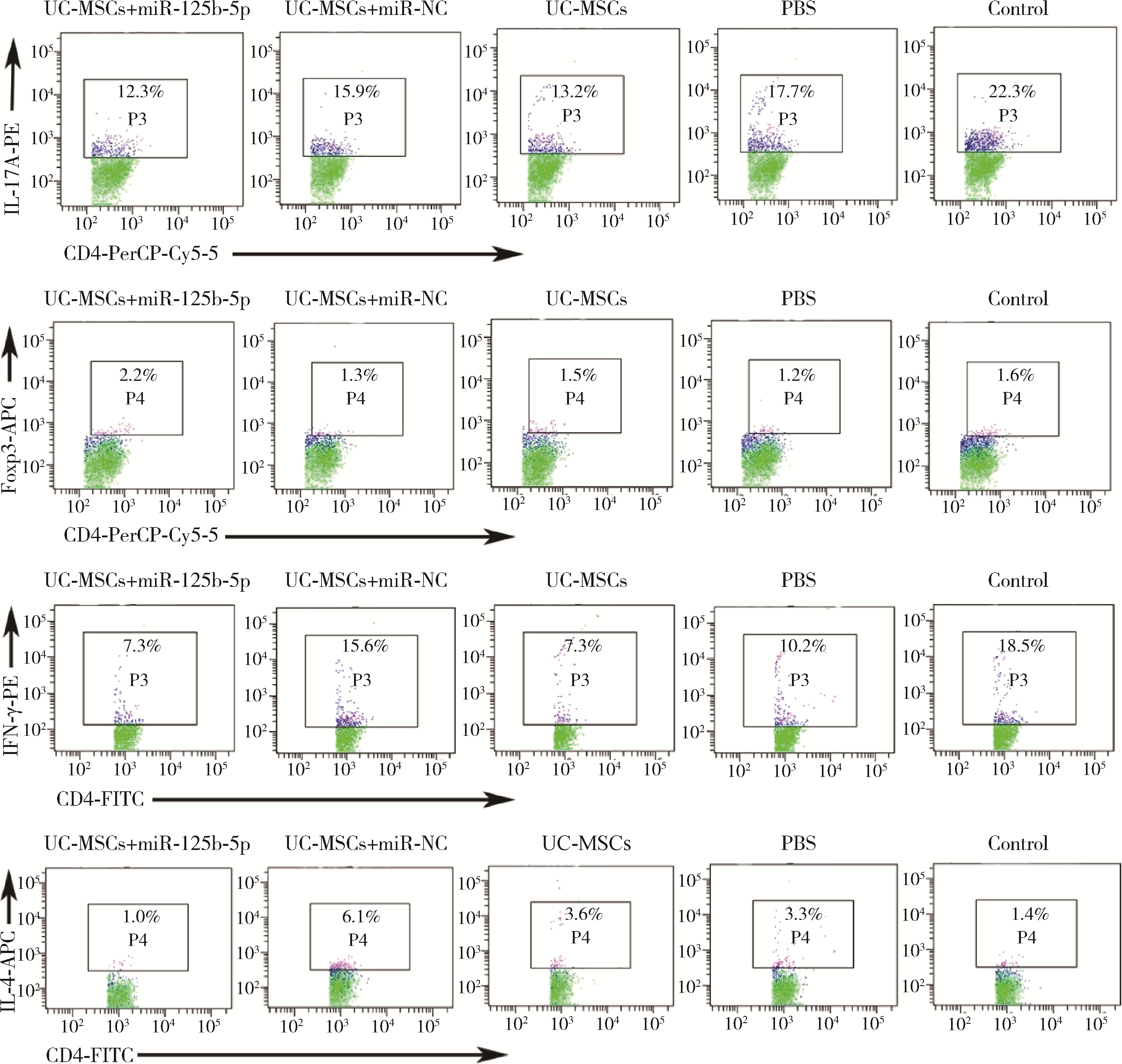
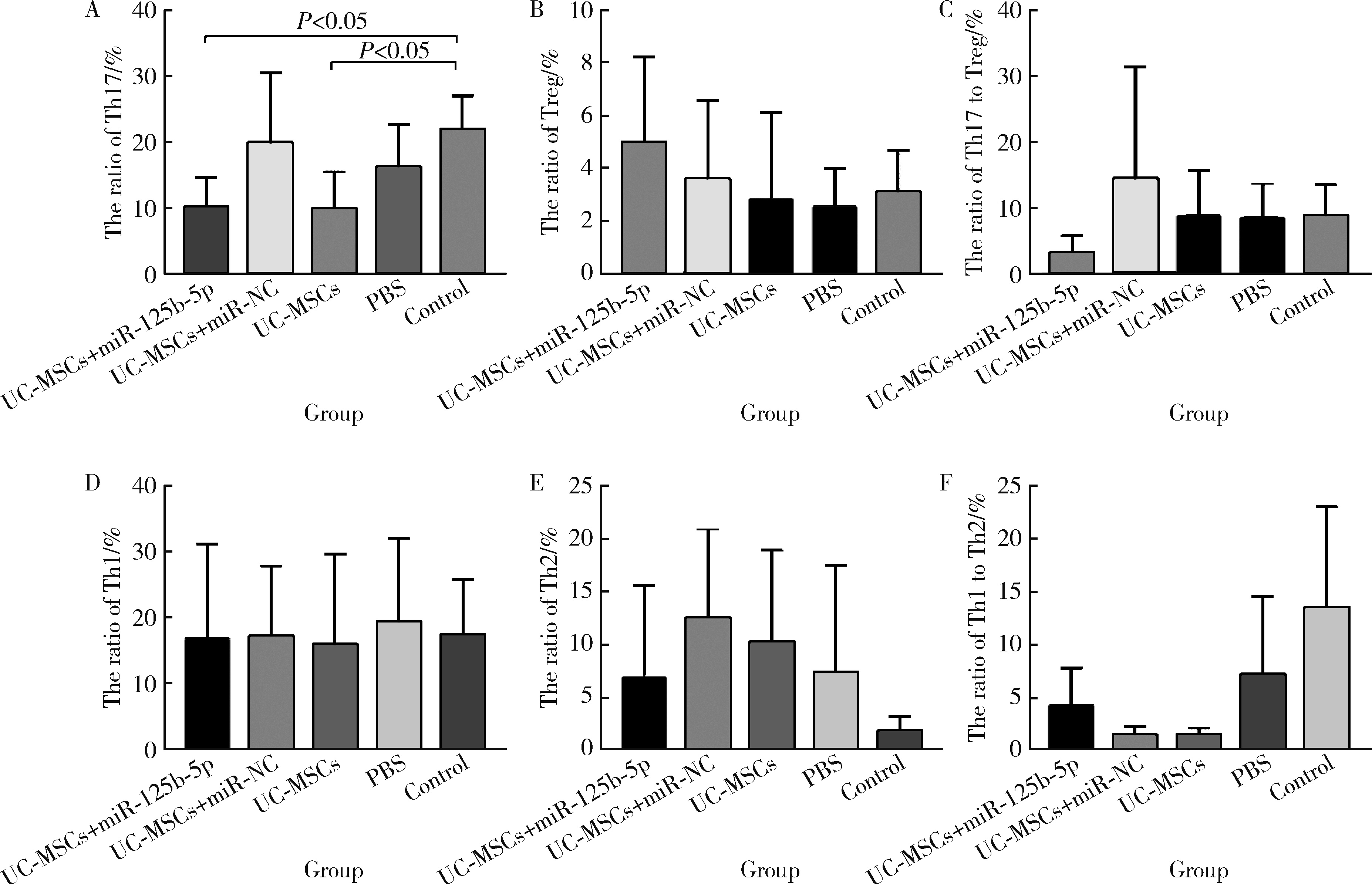
Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus
Zhihui WU1, Mingzhi HU1, Qiaoying ZHAO1, Fengfeng LV1, Jingying ZHANG2, Wei ZHANG1, Yongfu WANG2, Xiaolin SUN1,*( ), Hui WANG2,*(
), Hui WANG2,*( )
)
- 1. Central Laboratory, First Affiliated Hospital of Baotou Medical College (Inner Mongolia Key Laboratory of Autoimmunology), Baotou 014010, Inner Mongolia Autonomous Region, China
2. Department of Rheumatism and Immunology, First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia Autonomous Region, China
CLC Number:
- R593.2
| 1 |
Tsokos GC . Systemic lupus erythematosus[J]. N Engl J Med, 2011, 365 (22): 2110- 2121.
doi: 10.1056/NEJMra1100359 |
| 2 |
Relle M , Foehr B , Schwarting A . Epigenetic aspects of systemic lupus erythematosus[J]. Rheumatol Ther, 2015, 2 (1): 33- 46.
doi: 10.1007/s40744-015-0014-y |
| 3 |
Basta F , Fasola F , Triantafyllias K , et al. Systemic lupus erythematosus (SLE) therapy: The old and the new[J]. Rheumatol Ther, 2020, 7 (3): 433- 446.
doi: 10.1007/s40744-020-00212-9 |
| 4 |
Sharabi A , Tsokos GC . T cell metabolism: New insights in systemic lupus erythematosus pathogenesis and therapy[J]. Nat Rev Rheumatol, 2020, 16 (2): 100- 112.
doi: 10.1038/s41584-019-0356-x |
| 5 |
Muhammad Yusoff F , Wong KK , Mohd Redzwan N . Th1, Th2, and Th17 cytokines in systemic lupus erythematosus[J]. Autoimmunity, 2020, 53 (1): 8- 20.
doi: 10.1080/08916934.2019.1693545 |
| 6 |
Serakinci N , Fahrioglu U , Christensen R . Mesenchymal stem cells, cancer challenges and new directions[J]. Eur J Cancer, 2014, 50 (8): 1522- 1530.
doi: 10.1016/j.ejca.2014.02.011 |
| 7 |
Drela K , Lech W , Figiel-Dabrowska A , et al. Enhanced neuro-therapeutic potential of Wharton' s jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture[J]. Cytotherapy, 2016, 18 (4): 497- 509.
doi: 10.1016/j.jcyt.2016.01.006 |
| 8 |
Liu L , Wong CW , Han M , et al. Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis[J]. EBioMedicine, 2019, 47, 563- 577.
doi: 10.1016/j.ebiom.2019.08.073 |
| 9 |
Qi J , Tang X , Li W , et al. Mesenchymal stem cells inhibited the differentiation of MDSCs via COX2/PGE2 in experimental sialadenitis[J]. Stem Cell Res Ther, 2020, 11 (1): 325.
doi: 10.1186/s13287-020-01837-x |
| 10 | Liu C , Zhang H , Tang X , et al. Mesenchymal stem cells promote the osteogenesis in collagen-induced arthritic mice through the inhibition of TNF-α[J]. Stem Cells Int, 2018, 2018, 4069032. |
| 11 |
Choudhery MS , Badowski M , Muise A , et al. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation[J]. J Transl Med, 2014, 12, 8.
doi: 10.1186/1479-5876-12-8 |
| 12 | Escacena N , Quesada-Hernández E , Capilla-Gonzalez V , et al. Bottlenecks in the efficient use of advanced therapy medicinal products based on mesenchymal stromal cells[J]. Stem Cells Int, 2015, 2015, 895714. |
| 13 |
Fischer UM , Harting MT , Jimenez F , et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect[J]. Stem Cells Dev, 2009, 18 (5): 683- 692.
doi: 10.1089/scd.2008.0253 |
| 14 |
Abdelmohsen K , Gorospe M . Noncoding RNA control of cellular senescence[J]. Wiley Interdiscip Rev RNA, 2015, 6 (6): 615- 629.
doi: 10.1002/wrna.1297 |
| 15 | Su T , Xiao Y , Xiao Y , et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance[J]. ACS Nano, 2019, 13 (2): 2450- 2462. |
| 16 |
Meng Y , Eirin A , Zhu XY , et al. Micro-RNAs regulate metabolic syndrome-induced senescence in porcine adipose tissue-derived mesenchymal stem cells through the P16/MAPK pathway[J]. Cell Transplant, 2018, 27 (10): 1495- 1503.
doi: 10.1177/0963689718795692 |
| 17 | Vishnoi A , Rani S . MiRNA biogenesis and regulation of diseases: An overview[J]. Methods Mol Biol, 2017, 1509, 1- 10. |
| 18 | Gong B , Zheng L , Lu Z , et al. Mesenchymal stem cells negatively regulate CD4+ T cell activation in patients with primary Sjögren syndrome through the miRNA-125b and miRNA-155 TCR pathway[J]. Mol Med Rep, 2021, 23 (1): 43. |
| 19 |
Xiu L , Xing Q , Mao J , et al. miRNA-125b-5p suppresses hypo-thyroidism development by targeting signal transducer and activator of transcription 3[J]. Med Sci Monit, 2018, 24, 5041- 5049.
doi: 10.12659/MSM.907510 |
| 20 |
胡明智, 张晶莹, 杨国安, 等. miR-1-5p修饰脐带间充质干细胞对系统性红斑狼疮T淋巴细胞亚群的免疫调节[J]. 中国组织工程研究, 2021, 25 (31): 4928- 4938.
doi: 10.12307/2021.132 |
| 21 |
Gentile P , Sterodimas A . Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia[J]. Expert Opin Biol Ther, 2020, 20 (7): 711- 716.
doi: 10.1080/14712598.2020.1761322 |
| 22 |
Toyserkani NM , Jørgensen MG , Tabatabaeifar S , et al. Concise review: A safety assessment of adipose-derived cell therapy in cli-nical trials: A systematic review of reported adverse events[J]. Stem Cells Transl Med, 2017, 6 (9): 1786- 1794.
doi: 10.1002/sctm.17-0031 |
| 23 |
Chen C , Liang J , Yao G , et al. Mesenchymal stem cells upregulate Treg cells via sHLA-G in SLE patients[J]. Int Immuno-pharmacol, 2017, 44, 234- 241.
doi: 10.1016/j.intimp.2017.01.024 |
| 24 | 张立民. 系统性红斑狼疮microRNA表达谱和功能的初步研究[D]. 北京: 中国协和医科大学, 2010. |
| 25 |
Wang D , Huang S , Yuan X , et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus[J]. Mol Immunol, 2017, 14 (5): 423- 431.
doi: 10.1038/cmi.2015.89 |
| 26 |
Golpanian S , DiFede DL , Pujol MV , et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase Ⅰ/Ⅱ, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty[J]. Oncotarget, 2016, 7 (11): 11899- 11912.
doi: 10.18632/oncotarget.7727 |
| 27 |
Yang J , Yang X , Zou H , et al. Oxidative stress and Treg and Th17 dysfunction in systemic lupus erythematosus[J]. Oxid Med Cell Longev, 2016, 2016, 2526174.
doi: 10.1155/2016/2526174 |
| 28 |
Li D , Guo B , Wu H , et al. Interleukin-17 in systemic lupus erythematosus: A comprehensive review[J]. Autoimmunity, 2015, 48 (6): 353- 361.
doi: 10.3109/08916934.2015.1037441 |
| 29 |
Chen DY , Chen YM , Wen MC , et al. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis[J]. Lupus, 2012, 21 (13): 1385- 1396.
doi: 10.1177/0961203312457718 |
| 30 |
La Cava A . Tregs in SLE: An Update[J]. Curr Rheumatol Rep, 2018, 20 (2): 6.
doi: 10.1007/s11926-018-0714-8 |
| 31 |
Beringer A , Noack M , Miossec P . IL-17 in chronic inflammation: From discovery to targeting[J]. Trends Mol Med, 2016, 22 (3): 230- 241.
doi: 10.1016/j.molmed.2016.01.001 |
| 32 |
Dolff S , Bijl M , Huitema MG , et al. Disturbed Th1, Th2, Th17 and T(reg) balance in patients with systemic lupus erythematosus[J]. Clin Immunol, 2011, 141 (2): 197- 204.
doi: 10.1016/j.clim.2011.08.005 |
| 33 |
Moseley TA , Haudenschild DR , Rose L , et al. Interleukin-17 family and IL-17 receptors[J]. Cytokine Growth Factor Rev, 2003, 14 (2): 155- 174.
doi: 10.1016/S1359-6101(03)00002-9 |
| 34 | Qu N , Xu M , Mizoguchi I , et al. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases[J]. Clin Dev Immunol, 2013, 2013, 968549. |
| 35 |
Ghali JR , Holdsworth SR , Kitching AR . Targeting IL-17 and IL-23 in immune mediated renal disease[J]. Curr Med Chem, 2015, 22 (38): 4341- 4365.
doi: 10.2174/0929867322666151030163022 |
| 36 |
Talaat RM , Mohamed SF , Bassyouni IH , et al. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity[J]. Cytokine, 2015, 72 (2): 146- 153.
doi: 10.1016/j.cyto.2014.12.027 |
| 37 |
Zickert A , Amoudruz P , Sundström Y , et al. IL-17 and IL-23 in lupus nephritis: Association to histopathology and response to treatment[J]. BMC immunol, 2015, 16 (1): 7.
doi: 10.1186/s12865-015-0070-7 |
| 38 |
Jha AN , Singh VK , Kumari N , et al. IL-4 haplotype -590T, -34T and intron-3 VNTR R2 is associated with reduced malaria risk among ancestral indian tribal populations[J]. PLoS One, 2012, 7 (10): e48136.
doi: 10.1371/journal.pone.0048136 |
| 39 |
Patterson D , Jones C , Hart I , et al. The human interleukin-1 receptor antagonist (IL1RN) gene is located in the chromosome 2q14 region[J]. Genomics, 1993, 15 (1): 173- 176.
doi: 10.1006/geno.1993.1025 |
| 40 | Rapoport M , Bloch O . Systemic lupus erythematosus[J]. N Engl J Med, 2012, 366 (6): 574. |
| 41 |
Tsokos GC , Lo MS , Costa Reis P , et al. New insights into the immunopathogenesis of systemic lupus erythematosus[J]. Nat Rev Rheumatol, 2016, 12 (12): 716- 730.
doi: 10.1038/nrrheum.2016.186 |
| [1] | Hongyan WANG, Xinming LI, Kechi FANG, Huaqun ZHU, Rulin JIA, Jing WANG. Analysis of characteristics related to the disease activity of systemic lupus erythematosus and construction of an evaluation model [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1017-1022. |
| [2] | Dandan CHEN, Yun LI, Qingyi LU, Xiaohong XIANG, Feng SUN, Yingni LI, Jing ZHAO, Hongyan WANG, Chun LI. Ovarian function in patients of childbearing age with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1023-1028. |
| [3] | Li WANG, Chao GAO, Huanhuan REN, Yanping SHEN, Xiaowei HUANG, Hong YAO, Dandan HAN. Current status and influential factors of self-management ability in patients with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1029-1035. |
| [4] | Jing CHAI, Yue WANG, Rong MU, Jinxia ZHAO. Systemic lupus erythematosus involving the fornix column leading to hyponatremia: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1115-1118. |
| [5] | Mingxia WANG, Ling DING, Min WANG, Chanjuan ZOU, Siyu YAN, Yingwen LIANG, Weijia WANG, Shanzhi HE. Safe pregnancy and delivery in a female patient with systemic lupus erythematosus after discontinuation of dual-target chimeric antigen receptor T cells therapy [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1119-1125. |
| [6] | Limin REN,Chuchu ZHAO,Yi ZHAO,Huiqiong ZHOU,Liyun ZHANG,Youlian WANG,Lingxun SHEN,Wenqiang FAN,Yang LI,Xiaomei LI,Jibo WANG,Yongjing CHENG,Jiajing PENG,Xiaozhen ZHAO,Miao SHAO,Ru Li. Low disease activity and remission status of systemic lupus erythematosus in a real-world study [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 273-278. |
| [7] | Zhi-jun LUO,Jia-jia WU,You SONG,Chun-li MEI,Rong DU. Systemic lupus erythematosus associated macrophage activation syndrome with neuropsychiatric symptoms: A report of 2 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1111-1117. |
| [8] | Hai-hong YAO,Fan YANG,Su-mei TANG,Xia ZHANG,Jing HE,Yuan JIA. Clinical characteristics and diagnostic indicators of macrophage activation syndrome in patients with systemic lupus erythematosus and adult-onset Still's disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 966-974. |
| [9] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [10] | Lin-qi ZHANG,Jing ZHAO,Hong-yan WANG,Zong-yi WANG,Ying-ni LI,Ji-yang TANG,Si-ying LI,Jin-feng QU,Ming-wei ZHAO. Relationship between anti-ENO1 antibody and systemic lupus erythematosus patients with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1099-1105. |
| [11] | Min LI,Lin-qing HOU,Yue-bo JIN,Jing HE. Clinical and immunological characteristics of systemic lupus erythematosus with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1106-1111. |
| [12] | Miao SHAO,Hui-fang GUO,Ling-yan LEI,Qing ZHAO,Yan-jie DING,Jin LIN,Rui WU,Feng YU,Yu-cui LI,Hua-li MIAO,Li-yun ZHANG,Yan DU,Rui-ying JIAO,Li-xia PANG,Li LONG,Zhan-guo LI,Ru LI. A multicenter study on the tolerance of intravenous low-dose cyclophosphamide in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1112-1116. |
| [13] | Chao HAN,Zhu-xing ZHOU,You-rong CHEN,Zi-hui DONG,Jia-kuo YU. Biological characteristics of sheep peripheral blood mesenchymal stem cell [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1151-1157. |
| [14] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
| [15] | Jian-mei ZOU,Li-jun WU,Cai-nan LUO,Ya-mei SHI,Xue WU. Relationship of serum 25- hydroxy vitamin D and systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 938-941. |
|
||