Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (2): 262-266. doi: 10.19723/j.issn.1671-167X.2025.02.006
Previous Articles Next Articles
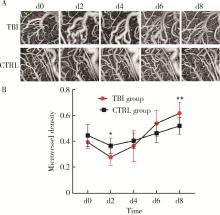
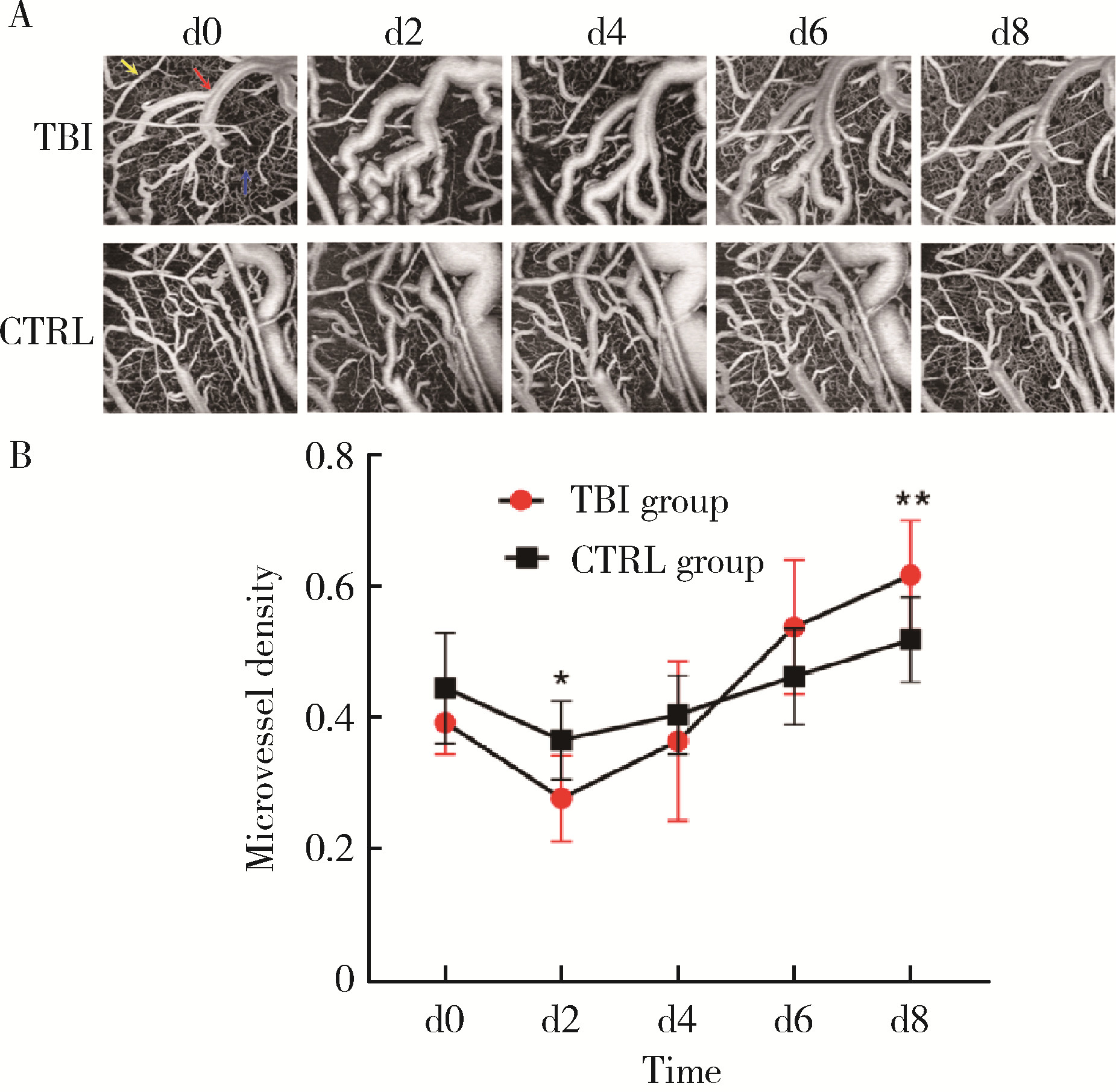
Optical coherence tomography angiography and microvessel density quantification in penumbra after traumatic brain injury in rats
Peng ZHONG1, Xiaodan HU2,3, Zhenzhou WANG3,*( )
)
- 1. Department of Ultrasound, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
2. School of Basic Medical Sciences, Peking University, Beijing 100191, China
3. Trauma Center, National Center for Trauma Medicine, Key Laboratory of Trauma and Neural Regeneration, Peking University People's Hospital, Beijing, 100044, China
CLC Number:
- R651.15
| 1 | Capizzi A , Woo J , Verduzco-Gutierrez M . Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management[J]. Med Clin North Am, 2020, 104 (2): 213- 238. |
| 2 | Graham DI , Adams JH . Ischaemic brain damage in fatal head injuries[J]. Lancet, 1971, 1 (7693): 265- 266. |
| 3 |
Sahuquillo J , Poca MA , Amoros S . Current aspects of patho-physiology and cell dysfunction after severe head injury[J]. Curr Pharm Des, 2001, 7 (15): 1475- 1503.
doi: 10.2174/1381612013397311 |
| 4 |
Demers-Marcil S , Coles JP . Cerebral metabolic derangements following traumatic brain injury[J]. Curr Opin Anaesthesiol, 2022, 35 (5): 562- 569.
doi: 10.1097/ACO.0000000000001183 |
| 5 |
Hays L , Udy A , Adamides AA , et al. Effects of brain tissue oxygen (PbtO2) guided management on patient outcomes following severe traumatic brain injury: A systematic review and meta-analysis[J]. J Clin Neurosci, 2022, 99, 349- 358.
doi: 10.1016/j.jocn.2022.03.017 |
| 6 |
Reddy L , Murugan D , Mullick M , et al. Recent approaches for angiogenesis in search of successful tissue engineering and rege-neration[J]. Curr Stem Cell Res Ther, 2020, 15 (2): 111- 134.
doi: 10.2174/1574888X14666191104151928 |
| 7 | Bragin DE , Bragina OA , Kameneva MV , et al. Resuscitation with drag reducing polymers after traumatic brain injury with hemor-rhagic shock reduces microthrombosis and oxidative stress[J]. Adv Exp Med Biol, 2020, 1232, 39- 45. |
| 8 |
de Carlo TE , Romano A , Waheed NK , et al. A review of optical coherence tomography angiography (OCTA)[J]. Int J Retina Vitreous, 2015, 1, 5.
doi: 10.1186/s40942-015-0005-8 |
| 9 |
Feeney DM , Boyeson MG , Linn RT , et al. Responses to cortical injury: Ⅰ. Methodology and local effects of contusions in the rat[J]. Brain Res, 1981, 211 (1): 67- 77.
doi: 10.1016/0006-8993(81)90067-6 |
| 10 |
Liu X , Huang Z , Wang Z , et al. A deep learning based pipeline for optical coherence tomography angiography[J]. J Biophotonics, 2019, 12 (10): e201900008.
doi: 10.1002/jbio.201900008 |
| 11 |
Wang Z , Liu J , Liu X , et al. Perfusion microvessel density in the cerebral cortex of septic rats is negatively correlated with endothe-lial microparticles in circulating plasma[J]. Metab Brain Dis, 2021, 36 (5): 1029- 1036.
doi: 10.1007/s11011-021-00702-x |
| 12 |
Veenith TV , Carter EL , Geeraerts T , et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury[J]. JAMA Neurol, 2016, 73 (5): 542- 550.
doi: 10.1001/jamaneurol.2016.0091 |
| 13 |
Sandsmark DK , Bashir A , Wellington CL , et al. Cerebral microvascular injury: A potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration[J]. Neuron, 2019, 103 (3): 367- 379.
doi: 10.1016/j.neuron.2019.06.002 |
| 14 | Kenney K , Amyot F , Haber M , et al. Cerebral vascular injury in traumatic brain injury[J]. Exp Neurol, 2016, 275 (Pt 3): 353- 366. |
| 15 | Manglani M , McGavern DB . Intravital imaging of neuroimmune interactions through a thinned skull[J]. Curr Protoc Immunol, 2018, 120, 24.2.1- 24.2.12. |
| 16 |
Hattori R , Komiyama T . Longitudinal two-photon calcium imaging with ultra-large cranial window for head-fixed mice[J]. STAR Protoc, 2022, 3 (2): 101343.
doi: 10.1016/j.xpro.2022.101343 |
| 17 | Qin D , Wang J , Le A , et al. Traumatic brain injury: Ultrastructural features in neuronal ferroptosis, glial cell activation and polarization, and blood-brain barrier breakdown[J]. Cells, 2021, 10 (5): 1009. |
| 18 | Grutzendler J , Murikinati S , Hiner B , et al. Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation[J]. Sci Transl Med, 2014, 6 (226): 226ra31. |
| 19 |
van der Wijk AE , Georgakopoulou T , Majolée J , et al. Microembolus clearance through angiophagy is an auxiliary mechanism preserving tissue perfusion in the rat brain[J]. Acta Neuropathol Commun, 2020, 8 (1): 195.
doi: 10.1186/s40478-020-01071-9 |
| 20 |
van der Wijk AE , Lachkar N , de Vos J , et al. Extravasation of microspheres in a rat model of silent brain infarcts[J]. Stroke, 2019, 50 (6): 1590- 1594.
doi: 10.1161/STROKEAHA.119.024975 |
| 21 | Park E , Bell JD , Siddiq IP , et al. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: A role for hypoxia-inducible factors in traumatic brain injury[J]. J Cereb Blood Flow Metab, 2009, 29 (3): 575- 584. |
| [1] | Shan YE,Ping-ping JIN,Nan ZHANG,Hai-bo WU,Lin SHI,Qiang ZHAO,Kun YANG,Hui-shu YUAN,Dong-sheng FAN. Cortical thickness and cognitive impairment in patients with amyotrophic lateral sclerosis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1158-1162. |
|
||