Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (1): 151-158. doi: 10.19723/j.issn.1671-167X.2019.01.026
Previous Articles Next Articles
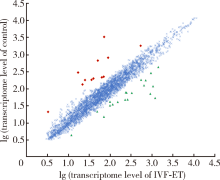
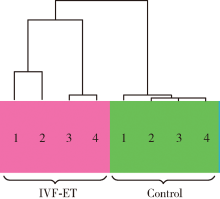
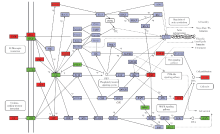
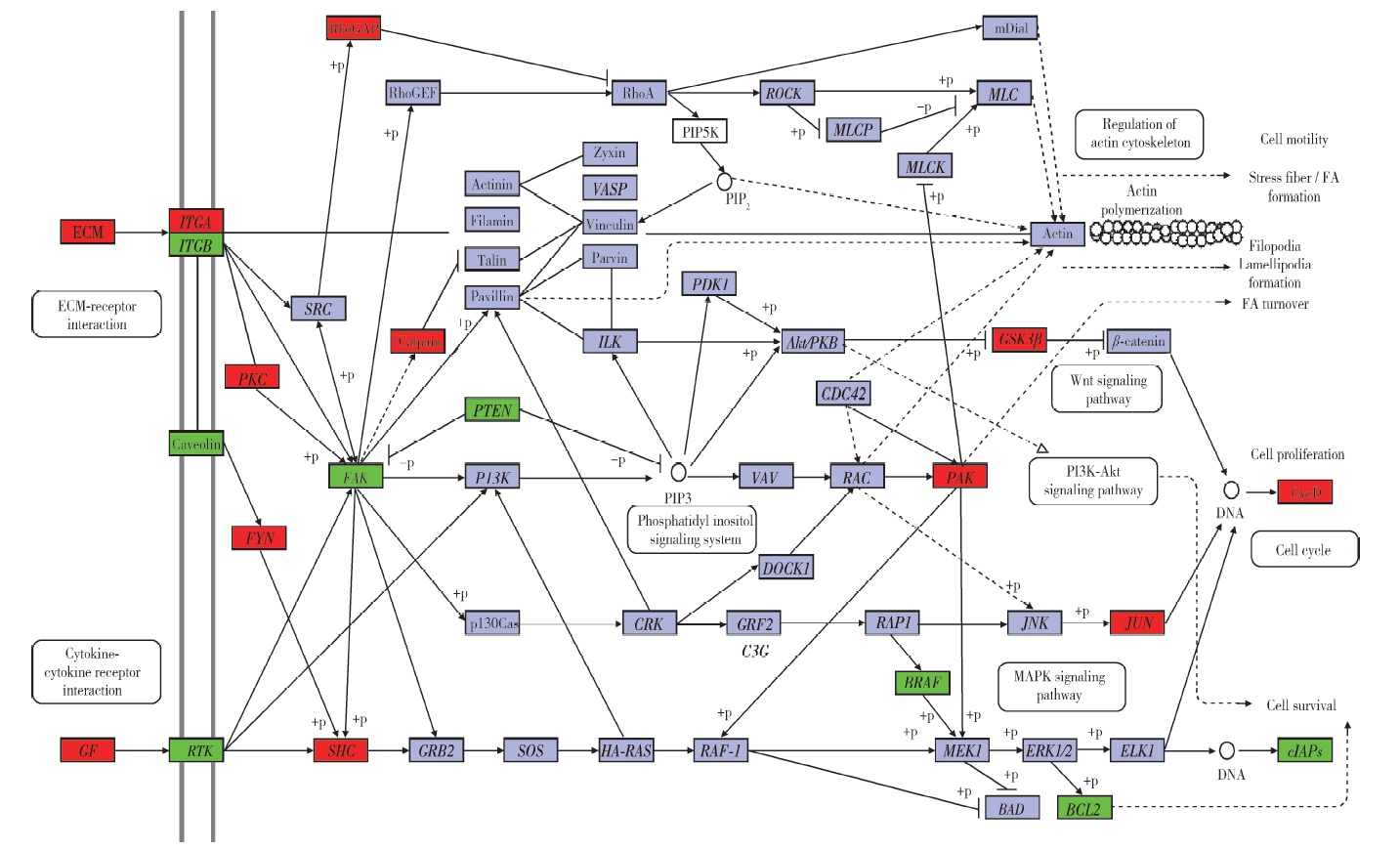
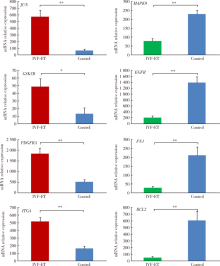
In vitro fertilization-embryo transfer affects focal adhension kinase signaling pathway in early placenta
Liang ZHAO1,△( ),Li-fang SUN1,Xiu-li ZHENG1,Jing-fang LIU1,Rong ZHENG1,Ying WANG2,Rui YANG2,Lei ZHANG3,Li YU4,Han ZHANG1
),Li-fang SUN1,Xiu-li ZHENG1,Jing-fang LIU1,Rong ZHENG1,Ying WANG2,Rui YANG2,Lei ZHANG3,Li YU4,Han ZHANG1
- 1. Department of Obstetrics and Gynecology, Beijing Jishuitan Hospital, Beijing 100035, China
2. Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing 100191, China
3. Department of Obstetrics and Gynecology, Beijing Tsinghua Changgung Hospital, Beijing 102218, China
4. Department of Obstetrics and Gynecology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R321-33
| [1] |
Ata B, Mumusoglu S, Aslan K , et al. Which is worse? A compa-rison of ART outcome between women with primary or recurrent endometriomas[J]. Hum Reprod, 2017,32(7):1539-1549.
doi: 10.1093/humrep/dex099 pmid: 28498960 |
| [2] |
Shapiro AJ, Darmon SK, Barad DH , et al. Effect of race and ethnicity on utilization and outcomes of assisted reproductive technology in the USA[J]. Reprod Biol Endocrinol, 2017,15(1):44-60.
doi: 10.1186/s12958-017-0262-5 pmid: 28595591 |
| [3] |
Luo WW, Zhao WW, Lu JJ , et al. Cucurbitacin B suppresses metastasis mediated by reactive oxygen species (ROS) via focal adhesion kinase (FAK) in breast cancer MDA-MB-231 cells[J]. Chin J Nat Med, 2018,16(1):10-19.
doi: 10.1016/S1875-5364(18)30025-6 pmid: 29425586 |
| [4] |
Zhang Y, Cui Y, Zhou Z , et al. Altered global gene expressions of human placentae subjected to assisted reproductive technology treatments[J]. Placenta, 2010,31(4):251-258.
doi: 10.1016/j.placenta.2010.01.005 pmid: 20116094 |
| [5] |
Sakian S, Louie K, Wong EC , et al. Altered gene expression of H19 and IGF2 in placentas from ART pregnancies[J]. Placenta, 2015,36(10):1100-1105.
doi: 10.1016/j.placenta.2015.08.008 pmid: 26386650 |
| [6] |
Zhan Q, Qi X, Wang N , et al. Altered methylations of H19, Snrpn, Mest and Peg3 are reversible by developmental reprogramming in kidney tissue of ICSI-derived mice[J]. Sci Rep, 2017,7(1):11936-11944.
doi: 10.1038/s41598-017-11778-w pmid: 28931827 |
| [7] |
Mohammed BT, Sontakke SD, Ioannidis J , et al. The adequate corpus luteum: miR-96 promotes luteal cell survival and progeste-rone production[J]. J Clin Endocrinol Metab, 2017,102(7):2188-2198.
doi: 10.1210/jc.2017-00259 pmid: 28368475 |
| [8] |
Herzog EM, Eggink AJ, Willemsen SP , et al. Early- and late-onset preeclampsia and the tissue-specific epigenome of the placenta and newborn[J]. Placenta, 2017,58:122-132.
doi: 10.1016/j.preghy.2017.07.075 pmid: 28962690 |
| [9] |
赵亮, 孙丽芳, 郑秀丽 , 等. 植入前囊胚滋养外胚层基因空间表达[J]. 北京大学学报(医学版), 2017,49(6):965-973.
doi: 10.3969/j.issn.1671-167X.2017.06.006 |
| [10] | Labarrere CA, DiCarlo HL, Bammerlin E , et al. Failure of phy-siologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta[J]. Am J Obstet Gynecol, 2017,216(3):287-297. |
| [11] |
Song GY, Na Q, Wang D , et al. Microarray expression profile of lncRNAs and mRNAs in the placenta of non-diabetic macrosomia[J]. J Dev Orig Health Dis, 2018,9(2):191-197.
doi: 10.1017/S2040174417000927 pmid: 29141697 |
| [12] |
Xin L, Xu B, Ma L , et al. Proteomics study reveals that the dysregulation of focal adhesion and ribosome contribute to early pregnancy loss[J]. Proteomics Clin Appl, 2016,10(5):554-563.
doi: 10.1002/prca.201500136 pmid: 26947931 |
| [13] |
Hilal T, Fauble V, Ketterling RP , et al. Myeloid neoplasm with eosinophilia associated with isolated extramedullary FIP1L1/PDGFRA rearrangement[J]. Cancer Genet, 2018,220(1):13-18.
doi: 10.1016/j.cancergen.2017.10.004 pmid: 29310833 |
| [14] |
Zhang A, Lakshmanan J, Motameni A , et al. MicroRNA-203 suppresses proliferation in liver cancer associated with PIK3CA, p38MAPK, c-Jun, and GSK3 signaling[J]. Mol Cell Biochem, 2018,441(1-2):89-98.
doi: 10.1007/s11010-017-3176-9 pmid: 28887744 |
| [15] |
Wu L, Hafiz MZ, Guan Y , et al. 17β-estradiol suppresses carboxylesterases by activating c-Jun/AP-1 pathway in primary human and mouse hepatocytes[J]. Eur J Pharmacol, 2018,15(8):98-107.
doi: 10.1016/j.ejphar.2017.11.036 pmid: 29175444 |
| [16] |
He Y, Bai J, Liu P , et al. miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus[J]. EXCLI J, 2017,16:1297-1307.
doi: 10.17179/excli2017-491 pmid: 29333131 |
| [17] |
Mathew SA, Chandravanshi B, Bhonde R . Hypoxia primed placental mesenchymal stem cells for wound healing[J]. Life Sci, 2017,182:85-92.
doi: 10.1016/j.lfs.2017.06.016 pmid: 28625360 |
| [18] |
Guo M, Zhao X, Yuan X , et al. Elevated microRNA-34a contri-butes to trophoblast cell apoptosis in preeclampsia by targeting BCL-2[J]. J Hum Hypertens, 2017,31(12):815-820.
doi: 10.1038/jhh.2017.65 pmid: 29022890 |
| [19] |
Jiang C, Xu M, Kuang X , et al. Treponema pallidum flagellins stimulate MMP-9 and MMP-13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes[J]. Exp Cell Res, 2017,361(1):46-55.
doi: 10.1016/j.yexcr.2017.09.040 pmid: 28982539 |
| [20] |
Choux C, Binquet C, Carmignac V , et al. The epigenetic control of transposable elements and imprinted genes in newborns is affec-ted by the mode of conception: ART versus spontaneous conception without underlying infertility[J]. Hum Reprod, 2018,33(2):331-340.
doi: 10.1093/humrep/dex366 pmid: 29237055 |
| [21] |
Nakamura K, Kusama K, Bai R , et al. Increase in complement iC3b is associated with anti-inflammatory cytokine expression during late pregnancy in mice[J]. PLoS One, 2017,12(5):8442-8456.
doi: 10.1371/journal.pone.0178442 pmid: 28542608 |
| [22] |
Song X, Rui C, Meng L , et al. Long non-coding RNA RPAIN regulates the invasion and apoptosis of trophoblast cell lines via complement protein C1q[J]. Oncotarget, 2017,8(5):7637-7646.
doi: 10.18632/oncotarget.13826 pmid: 28032589 |
| [23] |
Weinerman R, Ord T, Bartolomei MS , et al. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model[J]. Biol Reprod, 2017,97(1):133-142.
doi: 10.1093/biolre/iox067 pmid: 28859279 |
| [24] |
Martin E, Smeester L, Bommarito PA , et al. Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period[J]. Epigenomics, 2017,9(3):267-278.
doi: 10.2217/epi-2016-0132 pmid: 28234023 |
| [1] | Chun-mei ZHANG,Rui YANG,Rong LI,Jie QIAO,Hai-ning WANG,Ying WANG. Successful assisted reproductive technology treatment for a woman with 46XX-17α-hydroxylase deficiency: A case report [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 751-755. |
| [2] | ZHOU Chuan, MA Xue, XING Yun-kun, LI Lu-di, CHEN Jie, YAO Bi-yun, FU Juan-ling, ZHAO Peng. Exploratory screening of potential pan-cancer biomarkers based on The Cancer Genome Atlas database [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 602-607. |
| [3] | ZHANG Xiao-wei, LAN Ke, YANG Wen-bo, LI Qing, ZHAO Yong-ping, YIN Hua-qi, Kite Brandes, BAI Wen-jun, XU Tao. Expression and localization of transmembrane protein CMTM2 in human testis and sperm [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 575-579. |
| [4] | GUO Qian, CHEN Xu-yong, SU Yin. Interleukin-2 signaling pathway regulating molecules in systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2016, 48(6): 1100-1104. |
| [5] | REN Xiu-lian, LIU Ping, LIAN Ying, HUANG Jin, ZHENG Xiao-ying, ZHU Ya-ju, QIAO Jie. Effect of catheter choice during embryo transfer on the clinical outcome of in vitro fertilization-embryo transfer [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 905-909. |
| [6] | ZUO Na, LIANG Xiao, WANG Yi-Xian, SHEN Juan, WANG Xiao-Li, WANG Xiu-Xia. Influence of in vitro fertilization and embryo transfer on the physical and intellectual development of the children at pre-school age [J]. Journal of Peking University(Health Sciences), 2014, 46(6): 931-935. |
| [7] | MA Jing, PEI Xiao-Lei, ZHANG Yang, WANG Ying. Expression, purification and functional identification of human PSMP recombinant protein in Chinese hamster ovary cells [J]. Journal of Peking University(Health Sciences), 2014, 46(5): 669-675. |
| [8] | SHEN Juan,WANG Yi-xian, LIANG Xiao, WANG Xiu-xia. Comparison of clinical outcome of mild-stimulation and conventional ovarian stimulation in in vitro fertilization-embryo transfer (IVF-ET) [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 892-895. |
| [9] | CHEN Yuan, HAO Gui-min, WANG Xiu-xia, ZHANG Yun-shan, QIAO Jie, LIU Ping. Evaluation for the clinical application of all embryos cryopreservation: a multi-centre study in northern area of China [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 882-886. |
| [10] | REN Yun, YANG Shuo, YANG Rui, LI Rong, CHEN Xin-na, WANG Hai-yan, MA Cai-hong, LIU Ping, QIAO Jie. Comparison of gonadotropin releasing hormone agonist long protocol and gonadotropin releasing hormone antagonist protocol in infertile women [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 877-881. |
| [11] | HUANG Shuo, LI Rong, CHEN Xin-na, WANG Hai-yan, MA Cai-hong, LIU Ping, QIAO Jie. Influence of duration of gonadotropin administration on the clinical outcome of in vitro fertilization embryo transfer [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 873-876. |
|
||