Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (1): 145-150. doi: 10.19723/j.issn.1671-167X.2019.01.025
Previous Articles Next Articles
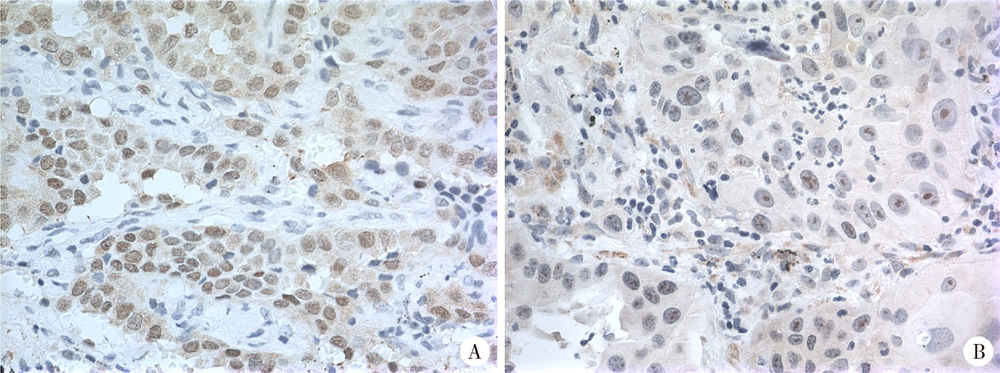
Expression of hUTP14a in non-small cell lung cancer
Chun-feng ZHANG1,Yun LIU2,Min LU3,Xiao-juan DU4,△( )
)
- 1. Department of Medical Genetics, Peking University School of Basic Medical Sciences, Beijing 100191, China
2. Peking University Centre of Medical and Health Analysis, Beijing 100191, China
3. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191, China
4. Department of Cell Biology, Peking University School of Basic Medical Sciences, Beijing 100191, China
CLC Number:
- R734.2
| [1] |
Zhang L, Li M, Wu N , et al. Time trends in epidemiologic cha-racteristics and imaging features of lung adenocarcinoma: a population study of 21,113 cases in China[J]. PLoS One, 2015,10(8):e0136727.
doi: 10.1371/journal.pone.0136727 pmid: 4552856 |
| [2] | 王媛媛, 毕玉, 王在翔 , 等. 山东省肺癌患者生存分析[J]. 中国卫生统计, 2018,35(1):111-116. |
| [3] |
黄文彦, 刘凯珊 . 以新视角观察p53家族在肺癌发生及治疗中的独特作用[J]. 中国肺癌杂志, 2013,16(8):422-425.
doi: 10.3779/j.issn.1009-3419.2013.08.06 |
| [4] |
李相国, 齐景宪, 易明福 . P16、Rb和PCNA在非小细胞肺癌的表达及临床意义[J]. 临床肺科杂志, 2008,13(8):1002-1004.
doi: 10.3969/j.issn.1009-6663.2008.08.023 |
| [5] |
Scheffner M, Huibregtse JM, Vierstra RD , et al. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53[J]. Cell, 1993,75(3):495-505.
doi: 10.1016/0092-8674(93)90384-3 pmid: 8221889 |
| [6] |
Uchida C, Miwa S, Kitagawa K , et al. Enhanced Mdm2 activity inhibits pRB function via ubiquitin-dependent degradation[J]. EMBO J, 2005,24(1):160-169.
doi: 10.1038/sj.emboj.7600486 pmid: 15577944 |
| [7] |
Sdek P, Ying H, Chang DL , et al. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein[J]. Mol Cell, 2005,20(5):699-708.
doi: 10.1016/j.molcel.2005.10.017 pmid: 16337594 |
| [8] |
Hu LL, Wang JN, Liu Y , et al. A small ribosomal subunit (SSU) processome component, the human U3 protein 14A (hUTP14A) binds p53 and promotes p53 degradation[J]. J Biol Chem, 2011,286(4):3119-3128.
doi: 10.1074/jbc.M110.157842 pmid: 21078665 |
| [9] |
Liu HJ, Wang JN, Liu Y , et al. Human U3 protein14a is a novel type ubiquitin ligase that binds RB and promotes RB degradation depending on a leucine-rich region[J]. Biochim Biophys Acta Mol Cell Res, 2018,1865(11 Pt A):1611-1620.
doi: 10.1016/j.bbamcr.2018.08.016 |
| [10] |
Zhang JY, Ren PW, Xu D , et al. Human UTP14a promotes colorectal cancer progression by forming a positive regulation loop with c-Myc [J]. Cancer Letters, 2018, 440- 441:106-115.
doi: 10.1016/j.canlet.2018.10.010 |
| [11] |
Zhang JY, Xu D, Liu ZZ , et al. Human U three protein 14a expression is increased in hepatocellular carcinoma and associated with poor prognosis[J]. Chin Med J (Engl), 2017,130(4):470-476.
doi: 10.4103/0366-6999.199839 pmid: 5324385 |
| [12] | Ma T, Lu CX, Guo YF , et al. Human U3 protein 14a plays an anti-apoptotic role in cancer cells[J]. Bio Chem, 2017,398(11):1247-1257. |
| [13] |
Groome PA, Bolejack V, Crowley JJ , et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours[J]. J Thorac Oncol, 2007,2(8):694-705.
doi: 10.1097/JTO.0b013e31812d05d5 pmid: 17762335 |
| [14] |
Zhang Y, Xiong Y, Yarbrough WG . ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways[J]. Cell, 1998,92(6):725-734.
doi: 10.1016/S0092-8674(00)81401-4 |
| [15] |
Leduc C, Claverie P, Eymin B , et al. p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation[J]. Oncogene, 2006,25(30):4147-4154.
doi: 10.1038/sj.onc.1209446 pmid: 16501607 |
| [16] |
Bozcuk H, Gumus A, Ozbilim G , et al. Cluster analysis of p-glycoprotein, c-erb-B2 and P53 in relation to tumor histology strongly indicates prognosis in patients with operable non-small cell lung cancer[J]. Med Sci Monit, 2005,11(6):11-20.
doi: 10.1051/medsci/2005216-7669 pmid: 15917726 |
| [17] |
张丽华, 侯振江 . p53在肺癌研究中的进展[J]. 临床肺科杂志, 2006,11(1):59-60.
doi: 10.3969/j.issn.1009-6663.2006.01.030 |
| [1] | Junqi SU,Xiaoying WANG,Zhiqiang SUN. Establishment and verification of a prognostic nomogram for survival of tongue squamous cell carcinoma patients who underwent cervical dissection [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 120-130. |
| [2] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [3] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [4] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [5] | Fang CAO,Ming ZHONG,Cong-rong LIU. Uterine POLE mutant endometrioid carcinoma combined with human papilloma virus-associated cervical adenocarcinoma: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 370-374. |
| [6] | ZHOU Chuan-xiang,ZHOU Zheng,ZHANG Ye,LIU Xiao-xiao,GAO Yan. Clinicopathological study in 28 cases of oral basaloid squamous cell carcinomas [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 62-67. |
| [7] | SU Jun-qi,SONG Yang,XIE Shang. Analysis of etiological characteristics and establishment of prediction model of postoperative infections in patients undergoing oral squamous cell carcinoma surgery with free flap reconstruction [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 68-76. |
| [8] | YU Yan-fei,HE Shi-ming,WU Yu-cai,XIONG Sheng-wei,SHEN Qi,LI Yan-yan,YANG Feng,HE Qun,LI Xue-song. Clinicopathological features and prognosis of fumarate hydratase deficient renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 640-646. |
| [9] | LIAO Xu-he,WANG Rong-fu,LIU Meng,CHEN Xue-qi,XIONG Yan,NONG Lin,YIN Lei,ZHANG Bing-ye,DU Yu-jing. Semiquantitative parameters of 18F-FDG PET/CT, gene mutation states of epidermal growth factor receptor and anaplastic lymphoma kinase in prognosis evaluation of patients with lung adenocarcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 246-254. |
| [10] | WANG Ying-chun,HUANG Yong-hui,CHANG Hong,YAO Wei,YAN Xiu-e,LI Ke,ZHANG Yao-peng,ZHENG Wei. Characteristics of benign and malignant lesions of ampullary polyps and the accuracy of forceps biopsy [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 204-209. |
| [11] | CHI Yan-ting,ZHANG Yan-ping,ZHANG Qiu-lu,LIU Cui-ling,LI bin-bin. Clinicopathological analysis of mucosa associated lymphoid tissue lymphoma secondary to Sjögren’s syndrome in salivary gland [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 40-45. |
| [12] | Lei-zhen SU,Jie CHEN,Xian LI,Ping JI. Effects of salinomycin on proliferation and apoptosis of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 902-906. |
| [13] | Ru MA,Xin-bao LI,Feng-cai YAN,Yu-lin LIN,Yan LI. Clinical evaluation of tumor-stroma ratio in pseudomyxoma peritonei from the appendix [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 240-246. |
| [14] | Xiao-peng ZHANG,Wei-yu ZHANG,Fei HUO,Hao HU,Qi WANG,Ke-xin XU. Outcome of surgical management and pathogenesis of female primary bladder neck obstruction [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1052-1055. |
| [15] | Chuan-si-bo TAO,Fan DONG,Dian-can WANG,Chuan-bin GUO. Diagnostic test for detection of cervical lymph node metastasis from oral squamous cell carcinoma via infrared thermal imaging [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 959-963. |
|
||