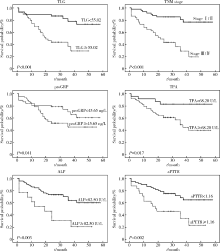
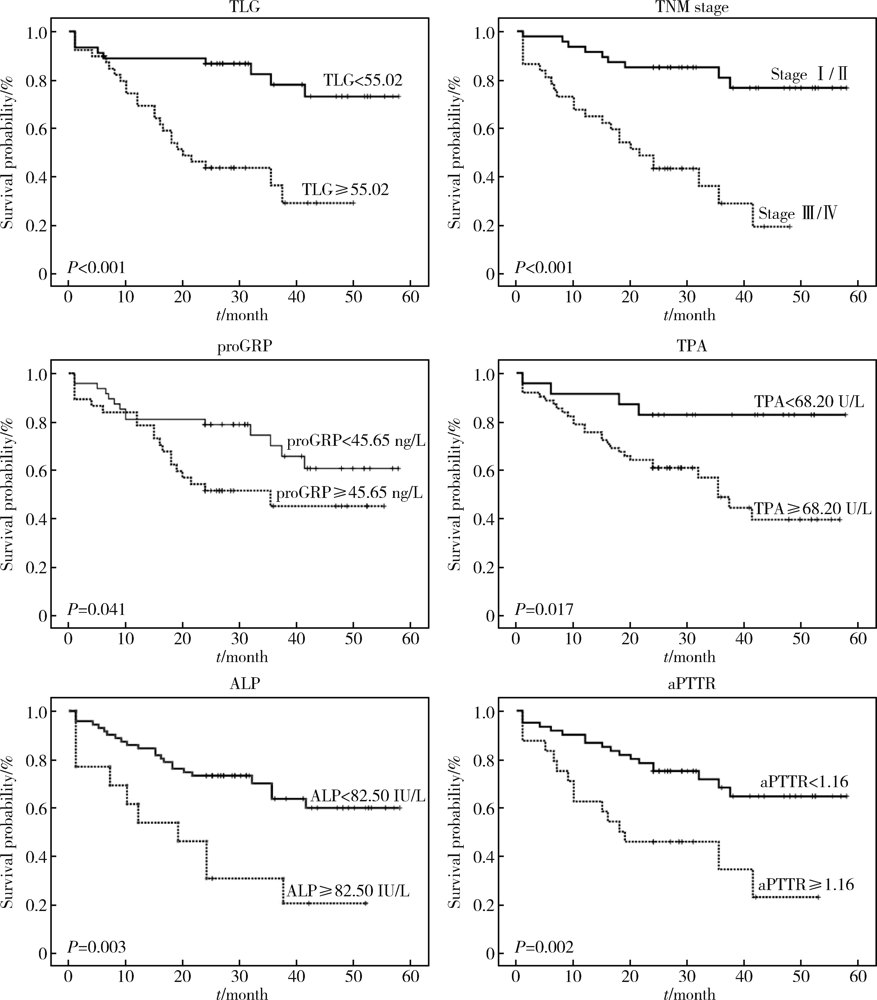
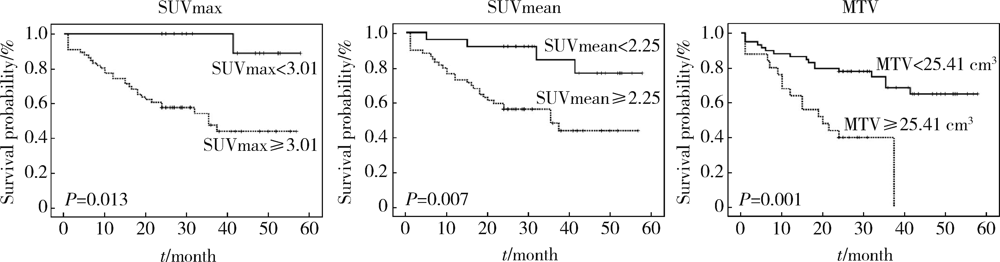
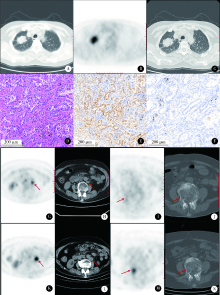
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 246-254. doi: 10.19723/j.issn.1671-167X.2021.02.003
Previous Articles Next Articles
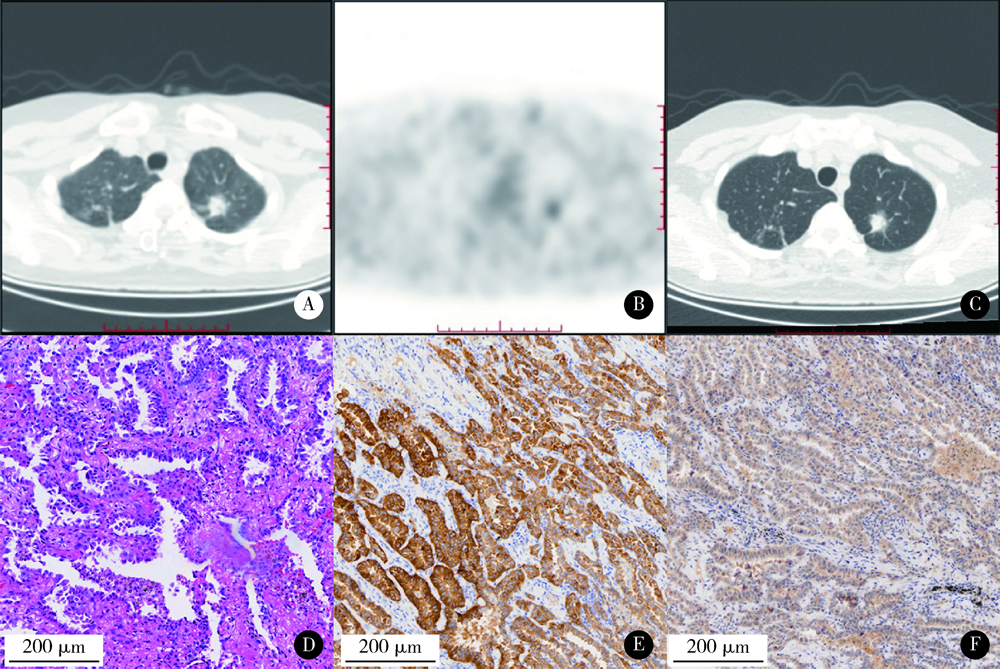
Semiquantitative parameters of 18F-FDG PET/CT, gene mutation states of epidermal growth factor receptor and anaplastic lymphoma kinase in prognosis evaluation of patients with lung adenocarcinoma
LIAO Xu-he1,WANG Rong-fu1,Δ( ),LIU Meng1,Δ(
),LIU Meng1,Δ( ),CHEN Xue-qi1,XIONG Yan2,NONG Lin2,YIN Lei1,ZHANG Bing-ye1,DU Yu-jing1
),CHEN Xue-qi1,XIONG Yan2,NONG Lin2,YIN Lei1,ZHANG Bing-ye1,DU Yu-jing1
- 1. Department of Nuclear Medicine, Peking University First Hospital, Beijing 100034, China
2. Department of Pathology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R734.2
| [1] | Noone A, Howlader N, Krapcho M, et al. SEER cancer statistics review (CRS), 1975-2015[EB/OL]. (2018-09-10) [2018-09-10]. https://seer.cancer.gov/csr/1975_2015/. |
| [2] |
Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress[J]. Chest, 2002,122(3):1037-1057.
doi: 10.1378/chest.122.3.1037 pmid: 12226051 |
| [3] |
Sharma A, Mohan A, Bhalla AS, et al. Role of various metabolic parameters derived from baseline 18F-FDG PET/CT as prognostic markers in non-small cell lung cancer patients undergoing platinum-based chemotherapy[J]. Clin Nucl Med, 2018,43(1):e8-e17.
pmid: 29112011 |
| [4] |
Salavati A, Duan F, Snyder BS, et al. Optimal FDG PET/CT volumetricparameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial[J]. Eur J Nucl Med Mol Imaging, 2017,44(12):1969-1983.
doi: 10.1007/s00259-017-3753-x pmid: 28689281 |
| [5] |
Wang WT, Li Y, Ma J, et al. Serum carcinoembryonic antigen levels before initial treatment are associated with EGFR mutations and EML4-ALK fusion gene in lung adenocarcinoma patients[J]. Asian Pac J Cancer Prev, 2014,15(9):3927-3932.
doi: 10.7314/apjcp.2014.15.9.3927 pmid: 24935562 |
| [6] |
Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma[J]. J Cancer Res Clin Oncol, 2014,140(1):89-98.
doi: 10.1007/s00432-013-1545-7 pmid: 24194352 |
| [7] |
Nisman B, Amir G, Lafair J, et al. Prognostic value of CYFRA 21-1, TPS and CEA in different histologic types of non-small cell lung cancer[J]. Anticancer Res, 1999,19(4C):3549-3552.
pmid: 10629651 |
| [8] |
Ma W, Wang M, Li X, et al. Quantitative 18F-FDG PET analysis in survival rate prediction of patients with non-small cell lung cancer[J]. Oncol Lett, 2018,16(4):4129-4136.
doi: 10.3892/ol.2018.9166 pmid: 30214552 |
| [9] |
Melloni G, Gajate AM, Sestini S, et al. New positron emission tomography derived parameters as predictive factors for recurrence in resected stage Ⅰ non-small cell lung cancer[J]. Eur J Surg Oncol, 2013,39(11):1254-1261.
doi: 10.1016/j.ejso.2013.07.092 pmid: 23948705 |
| [10] |
Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection[J]. Ann Surg, 2013,257(2):364-370.
pmid: 22968069 |
| [11] |
Kurtipek E, Cayci M, Duzgun N, et al. 18F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer[J]. Clin Nucl Med, 2015,40(6):459-463.
doi: 10.1097/RLU.0000000000000740 pmid: 25742234 |
| [12] |
Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed[J]. J Thorac Oncol, 2011,6(4):774-780.
pmid: 21336183 |
| [13] |
Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage Ⅰ non-small cell lung cancers[J]. Ann Thorac Surg, 2013,96(3):962-968.
pmid: 23932319 |
| [14] |
Nisman B, Biran H, Ramu N, et al. The diagnostic and prognostic value of ProGRP in lung cancer[J]. Anticancer Res, 2009,29(11):4827-4832.
pmid: 20032442 |
| [15] |
Cistaro A, Quartuccio N, Mojtahedi A, et al. Prediction of 2 years-survival in patients with stage Ⅰ and Ⅱ non-small cell lung cancer utilizing (18)F-FDG PET/CT SUV quantification[J]. Radiol Oncol, 2013,47(3):219-223.
pmid: 24133385 |
| [16] |
Soussan M, Chouahnia K, Maisonobe JA, et al. Prognostic implications of volume-based measurements on FDG PET/CT in stage Ⅲ non-small-cell lung cancer after induction chemotherapy[J]. Eur J Nucl Med Mol Imaging, 2013,40(5):668-676.
pmid: 23306807 |
| [17] | Liu J, Dong M, Sun X, et al. Prognosticvalue of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis[J]. PLoS One, 2016,11(1):e146195. |
| [18] |
Ohtaka K, Hida Y, Kaga K, et al. Outcome analysis of 18F-fluorodeoxyglucose positron-emission tomography in patients with lung cancer after partial volume correction[J]. Anticancer Res, 2013,33(11):5193-5198.
pmid: 24222169 |
| [19] |
Caicedo C, Garcia-Velloso MJ, Lozano MD, et al. Role of 18F-FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer[J]. Eur J Nucl Med Mol Imaging, 2014,41(11):2058-2065.
pmid: 24990403 |
| [20] |
Liu A, Han A, Zhu H, et al. The role of metabolic tumor volume (MTV) measured by [18F] FDG PET/CT in predicting EGFR gene mutation status in non-small cell lung cancer[J]. Oncotarget, 2017,8(20):33736-33744.
pmid: 28422710 |
| [21] | Li Q, Zhang J, Cheng W, et al. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis[J]. Medicine (Baltimore), 2017,96(37):e8084. |
| [22] |
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma[J]. N Engl J Med, 2009,361(10):947-957.
pmid: 19692680 |
| [23] |
Koizumi T, Fukushima T, Gomi D, et al. Correlation of early PET findings with tumor response to molecular targeted agents in patients with advanced driver-mutated non-small cell lung cancer[J]. Med Oncol, 2017,34(10):169.
doi: 10.1007/s12032-017-1032-0 pmid: 28864950 |
| [24] |
Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer[J]. Anticancer Res, 1999,19(4C):3613-3618.
pmid: 10629660 |
| [25] |
Alatas F, Alatas O, Metintas M, et al. Usefulness of bone mar-kers for detection of bone metastases in lung cancer patients[J]. Clin Biochem, 2002,35(4):293-296.
pmid: 12135691 |
| [26] |
Qi Y, Fu J. Research on the coagulation function changes in non small cell lung cancer patients and analysis of their correlation with metastasis and survival[J]. J BUON, 2017,22(2):462-467.
pmid: 28534370 |
| [27] | Ettinger DS, Wood DE, Aisner DL, et al. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: non-small cell lung cancer (2019V2)[EB/OL]. (2018-11-21) [2018-11-21]. https://www.nccn.org/professionals/physician_gls/default.aspx. |
| [1] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [2] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [3] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [4] | Fang CAO,Ming ZHONG,Cong-rong LIU. Uterine POLE mutant endometrioid carcinoma combined with human papilloma virus-associated cervical adenocarcinoma: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 370-374. |
| [5] | Yue WANG,Shuang ZHANG,Hong ZHANG,Li LIANG,Ling XU,Yuan-jia CHENG,Xue-ning DUAN,Yin-hua LIU,Ting LI. Clinicopathological features and prognosis of hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 853-862. |
| [6] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [7] | WANG Ying-chun,HUANG Yong-hui,CHANG Hong,YAO Wei,YAN Xiu-e,LI Ke,ZHANG Yao-peng,ZHENG Wei. Characteristics of benign and malignant lesions of ampullary polyps and the accuracy of forceps biopsy [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 204-209. |
| [8] | Yi BAO,Juan-fen MO. Concordant point mutation of ETS-related gene (ERG) in tumor tissues from a synchronous multiple primary lung cancer: A case report [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 971-974. |
| [9] | Liang GENG,Jing LV,Jing FAN. Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 247-253. |
| [10] | Xue-jun LIANG,Li-ying GONG,Fei ZHOU,De-min ZHOU,Jing-jing ZHU. Pharmacological effects of site specific conjugated anti-human epidermal growth factor receptor 2-antibody drug conjugate using unnatural amino acid technology [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 797-804. |
| [11] | Chun-feng ZHANG,Yun LIU,Min LU,Xiao-juan DU. Expression of hUTP14a in non-small cell lung cancer [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 145-150. |
| [12] | QIN Zi-jian, BI Hai, MA Lu-lin, HUANG Yi, ZHANG Fan. A primary intestinal-derived adenocarcinoma in intestine bladder substitutes:a case report [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 737-739. |
| [13] | ZHANG Xiao-dong, LIU De-ruo. Correlation between the new lung adenocarcinoma classification and epidermal growth factor receptor mutation [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 640-644. |
| [14] | ZHAO Yi-guo, YANG Xiao-dong, ZHANG Yan-kai, NING Ning, XING Zhao-dong, YE Ying-jiang. #br# Adenocarcinoma in a Meckel’s diverticulum with multiple liver metastases and gastrointestinal hemorrhage: a case report [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1095-1097. |
| [15] | MEI Fang, ZHAO Ting-ting, GAO Fei, ZHENG Jie. A rare pulmonary benign bi-phasic tumor: a case report of pulmonary adenofibroma and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1076-1080. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 261
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 889
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||