Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (4): 750-757. doi: 10.19723/j.issn.1671-167X.2021.04.022
Previous Articles Next Articles
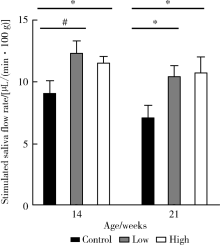
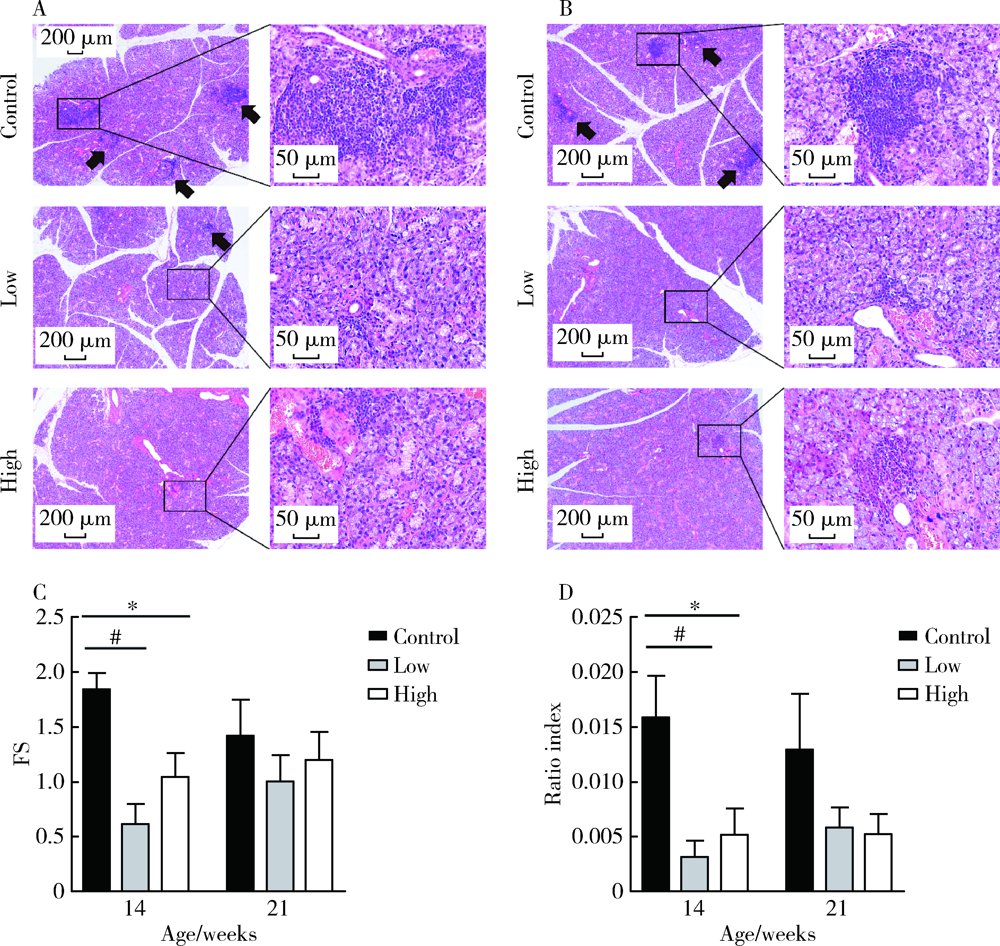
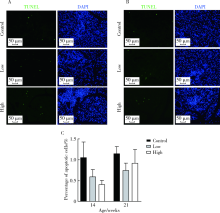
Effect of topical injection of cyclosporine A on saliva secretion and inflammation in the submandibular gland of non-obese diabetic mice
ZHU Yi-ying,MIN Sai-nan,YU Guang-yan( )
)
- Department of Oral and Maxilloficial Surgery, Peking University School and Hospital of Stomatology & National Center of Stomatology &National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R782.3
| [1] |
Ramos-Casals M, Tzioufas AG, Stone JH, et al. Treatment of primary Sjögren syndrome: a systematic review [J]. JAMA, 2010, 304(4):452-460.
doi: 10.1001/jama.2010.1014 pmid: 20664046 |
| [2] |
Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome [J]. Nat Rev Rheumatol, 2016, 12(8):456-471.
doi: 10.1038/nrrheum.2016.100 pmid: 27411907 |
| [3] | 苏运超, 吴立玲, 向若兰. 舍格伦综合征发病机制的实验研究 [J]. 生理科学进展, 2012, 43(3):13-18. |
| [4] | Scuron MD, Fay B, Oliver J, et al. Spontaneous model of Sjögren’s syndrome in NOD mice [J]. Cur protoc pharmacol, 2019, 86(1):e65. |
| [5] |
Lallemand F, Schmitt M, Bourges JL, et al. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts [J]. Eur J Pharm Biopharm, 2017, 117:14-28.
doi: S0939-6411(16)30908-0 pmid: 28315447 |
| [6] |
Chighizola CB, Ong VH, Meroni PL. The use of cyclosporine A in rheumatology: A 2016 comprehensive review [J]. Clin Rev Allergy Immunol, 2017, 52(3):401-423.
doi: 10.1007/s12016-016-8582-3 |
| [7] |
Brito-Zerón P, Retamozo S, Kostov B, et al. Efficacy and safety of topical and systemic medications: A systematic literature review informing the EULAR recommendations for the management of Sjögren’s syndrome [J]. RMD Open, 2019, 5(2):e001064.
doi: 10.1136/rmdopen-2019-001064 |
| [8] |
Guo YF, Sun NN, Wu CB, et al. Sialendoscopy-assisted treatment for chronic obstructive parotitis related to Sjögren’s syndrome [J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2017, 123(3):305-309.
doi: 10.1016/j.oooo.2016.10.011 |
| [9] | Nagy C, Einwallner E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) [J]. J Vis Exp, 2018(131):56672. |
| [10] |
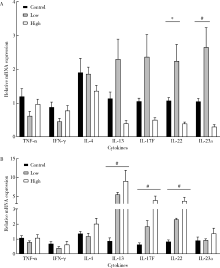
Borg DJ, Wang R, Murray L, et al. The effect of interleukin-22 treatment on autoimmune diabetes in the NOD mouse [J]. Diabetologia, 2017, 60(11):2256-2261.
doi: 10.1007/s00125-017-4392-2 |
| [11] |
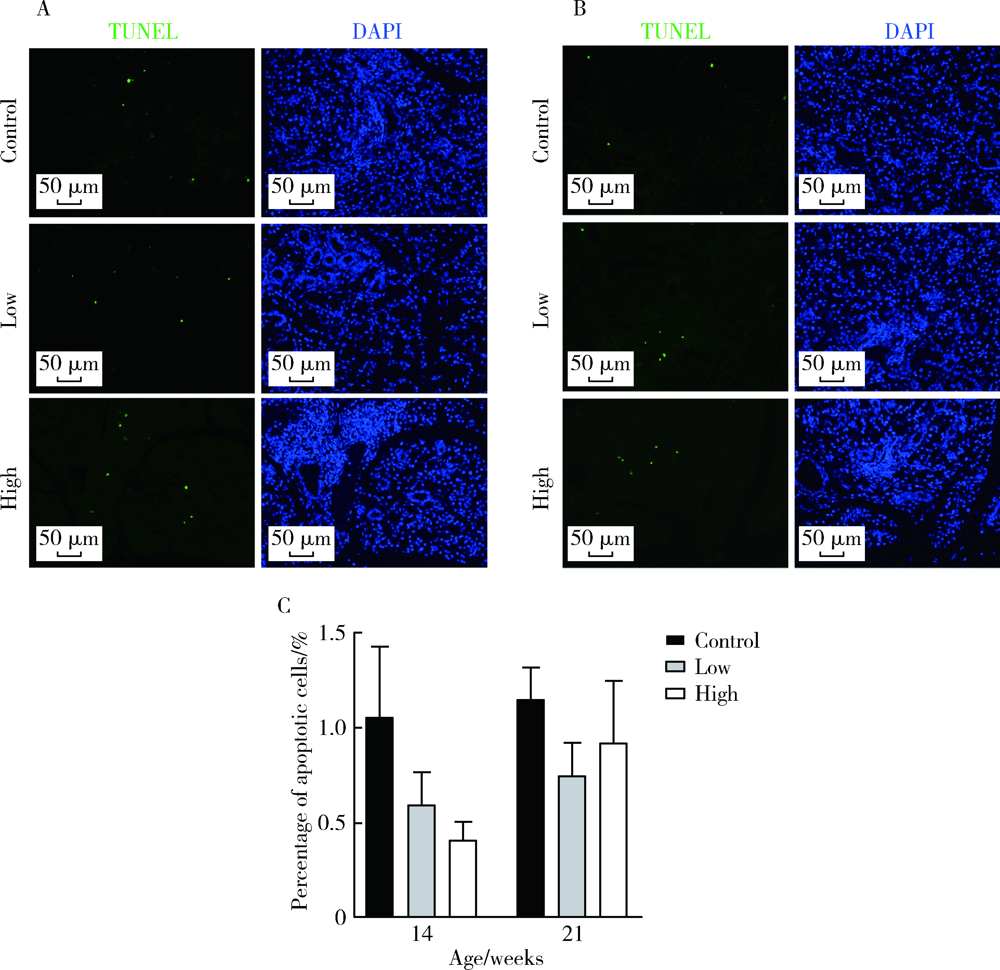
Kawai K, Watabe S, Matsuda M, et al. Correlation between expression of orotate phosphoribosyl transferase and 5-fluorouracil sensitivity, as measured by apoptosis index in colorectal cancer tissue [J]. Int J Gastrointest Cancer, 2005, 35(3):197-203.
pmid: 16110121 |
| [12] |
Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts [J]. Arthritis Rheumatol, 2017, 69(1):35-45.
doi: 10.1002/art.v69.1 |
| [13] |
Navarro-Mendoza EP, Aguirre-Valencia D, Posso-Osorio I, et al. Cytokine markers of B lymphocytes in minor salivary gland infiltrates in Sjögren’s syndrome [J]. Autoimmun Rev, 2018, 17(7):709-714.
doi: S1568-9972(18)30112-5 pmid: 29729452 |
| [14] |
Reff ME, Carner K, Chambers S, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20 [J]. Blood, 1994, 83(2):435-445.
pmid: 7506951 |
| [15] |
Blokland SCV, Versnel MA. Pathogenesis of Sjgren’s syndrome: Characteristics of different mouse models for autoimmune exocrinopathy [J]. Clin Immunol, 2002, 103(2):111-124.
pmid: 12027416 |
| [16] | Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjögren’s syndrome[J/OL]. J Biomed Biotechnol, 2011: 549107. (2010-12-30)[2020-10-30]. https://pubmed.ncbi.nlm.nih.gov/21253584 . |
| [17] |
Faulds D, Goa KL, Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders [J]. Drugs, 1993, 45(6):953-1040.
doi: 10.2165/00003495-199345060-00007 pmid: 7691501 |
| [18] |
Graham RM. Cyclosporine: mechanisms of action and toxicity [J]. Cleve Clin J Med, 1994, 61(4):308-313.
doi: 10.3949/ccjm.61.4.308 |
| [19] | United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers[EB/OL]. (2005-07-06)[2020-2-19].https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers . |
| [20] |
Daull P, Barabino S, Feraille L, et al. Modulation of inflammation-related genes in the cornea of a mouse model of dry eye upon treatment with cyclosporine eye drops [J]. Curr Eye Res, 2019, 44(5):476-485.
doi: 10.1080/02713683.2018.1563197 |
| [21] | Zhang FD, Hao ZQ, Gao W, et al. Effect of topical 0.05% cyclosporine A on the tear protein lacritin in a rat model of dry eye [J]. Int J Ophthalmol, 2019, 12(2):189-193. |
| [1] | Yushu YANG, Xuan QI, Meng DING, Wei WANG, Huifang GUO, Lixia GAO. Diagnostic values of anti-salivary gland protein-1 antibody combined with anti-parotid secretory protein antibody for Sjögren's syndrome [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 845-852. |
| [2] | Deng-gao LIU,Dan-ni ZHENG,Ya-ning ZHAO,Ya-qiong ZHANG,Xin YE,Li-qi ZHANG,Xiao-yan XIE,Lei ZHANG,Zu-yan ZHANG,Guang-yan YU. Recent progress in the treatment of intractable sialolithiasis [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 8-12. |
| [3] | Yang LIU,Fang CHENG,Yan-ling WANG,Xiang-yan AI,Zhen-hang ZHU,Fu-tao ZHAO. Diagnostic performances of salivary gland ultrasonography for Sjögren's syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1123-1127. |
| [4] | Guang-yan YU,Jia-zeng SU,Deng-gao LIU,Li-ling WU,Xin CONG. Establishment and application of new techniques for submandibular gland preservation [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 842-845. |
| [5] | CHEN Chao-lun,SU Jia-zeng,YU Guang-yan. Effects of acid stimulation on saliva flow rate and compositions of human parotid and submandibular glands [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 89-94. |
| [6] | YU Guang-yan,LIU Deng-gao,LI Wei,HONG Xia,ZHANG Yan-yan,ZHU Wen-xuan,ZHANG Ke-fu,LI Xiao,LI Zhan-guo,LIU Yan-ying,CHEN Yan,GAO Yan,SU Jia-zeng. Studies on newly recognized chronic sialadenitis [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 13-17. |
| [7] | WANG Yi-ping,CAI Zhi-gang,PENG Xin,ZHANG Jie,SUN Zhi-peng,LI Wei,ZHANG Lei,YU Guang-yan. Measurement of the weight and volume of submandibular gland in vitro [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 126-132. |
| [8] | Huan-bin YU,Wen-jie WU,Xiao-ming LV,Yan SHI,Lei ZHENG,Jian-guo ZHANG. 125I seed brachytherapy for recurrent salivary gland carcinoma after external radiotherapy [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 919-923. |
| [9] | Ye ZHANG,Ni ZHANG,Xiao-xiao LIU,Chuan-xiang ZHOU. Cervical lymph node metastasis in adenoid cystic carcinoma of the salivary glands: A clinicopathologic study [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 30-34. |
| [10] | Xin CONG,Sai-nan MIN,Li-ling WU,Zhi-gang CAI,Guang-yan YU. Role and mechanism of muscarinic acetylcholine receptor in the regulation of submandibular gland secretion [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 390-396. |
| [11] | Guang-ya YU,Xia HONG,Wei LI,Yan-yan ZHANG,Yan GAO,Yan CHEN,Zu-yan ZHANG,Xiao-yan XIE,Zhan-guo LI,Yan-ying LIU,Jia-zeng SU,Wen-xuan ZHU,Zhi-peng SUN. Clinicopathological characteristics and diagnosis of IgG4-related sialadenitis [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 1-3. |
| [12] | ZHANG Ya-qiong, YE Xin, LIU Deng-gao, ZHAO Ya-ning, XIE Xiao-yan, YU Guang-yan. Endoscopy-assisted sialodochoplasty for the treatment of severe sialoduct stenosis [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 160-164. |
| [13] | YU Guang-yan, WU Li-ling, CAI Zhi-gang, LV Lan, CONG Xin. A 20-year study on microvascular autologous transplantation of submandibular gland for treatment of severe dry eye [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 1-4. |
| [14] | WANG Wei, ZHENG Lei, LIU Shu-ming, HUANG Ming-wei, SHI Yan, LV Xiao-ming, ZHANG Jie, ZHANG Jian-guo. Distant metastases of malignant salivary gland carcinoma after treated by 125Ⅰinternal brachy therapy alone [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 547-550. |
| [15] | LI Qiong, CHANG Zhi-fang, YANG Guo-an, PANG Chun-yan, WANG Yong-fu. Effect of type 1 sphingosine-1-phosphate receptor siRNA on human salivary gland cells [J]. Journal of Peking University(Health Sciences), 2016, 48(6): 987-993. |
|
||