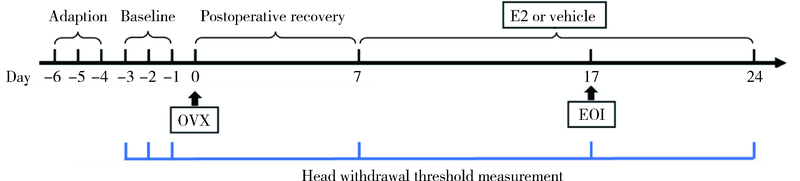
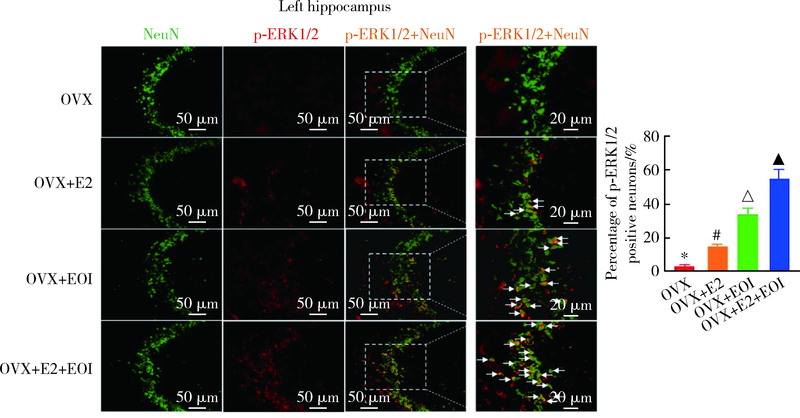
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (1): 40-47. doi: 10.19723/j.issn.1671-167X.2022.01.007
Previous Articles Next Articles
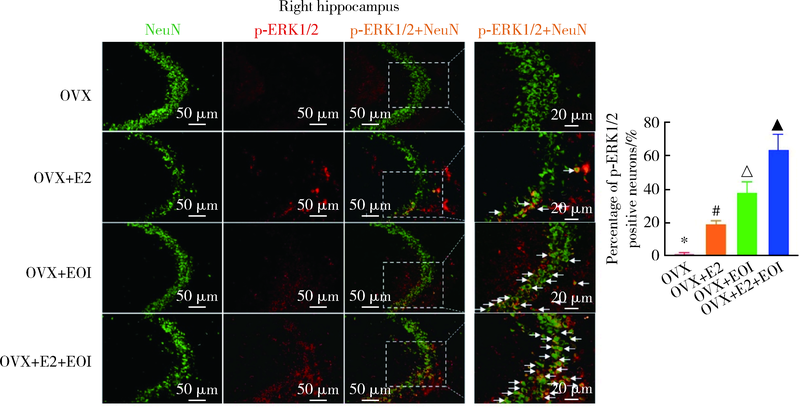
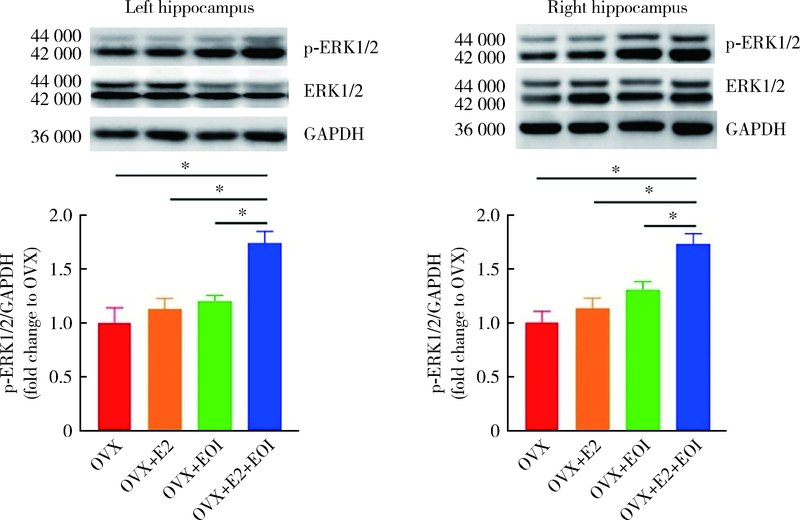
Hippocampus is involved in 17β-estradiol exacerbating experimental occlusal inter-ference-induced chronic masseter hyperalgesia in ovariectomized rats
FAN Ying-ying,LIU Yun,CAO Ye( ),XIE Qiu-fei(
),XIE Qiu-fei( )
)
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
CLC Number:
- R781.6
| [1] | Cao Y. Occlusal disharmony and chronic oro-facial pain: from clinical observation to animal study[J]. J Oral Rehabil, 2021, 7 (2021-07-17) [2021-09-19]. https://onlinelibrary.wiley.com/doi/epdf/10.1111/joor.13236 . |
| [2] | Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon[J]. Nat Rev Neuro-sci, 2012, 13(12):859-866. |
| [3] |
Cairns BE. The influence of gender and sex steroids on craniofacial nociception[J]. Headache, 2007, 47(2):319-324.
pmid: 17300382 |
| [4] |
Bueno CH, Pereira DD, Pattussi MP, et al. Gender differences in temporomandibular disorders in adult populational studies: a systematic review and meta-analysis[J]. J Oral Rehabil, 2018, 45(9):720-729.
doi: 10.1111/joor.12661 pmid: 29851110 |
| [5] | Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study[J]. J Pain, 2011, 12(11):12-26. |
| [6] |
Castrillon EE, Cairns BE, Wang K, et al. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women[J]. Pain, 2012, 153(4):823-829.
doi: 10.1016/j.pain.2012.01.003 pmid: 22336721 |
| [7] |
Cao Y, Xie QF, Li K, et al. Experimental occlusal interference induces long-term masticatory muscle hyperalgesia in rats[J]. Pain, 2009, 144(3):287-293.
doi: 10.1016/j.pain.2009.04.029 pmid: 19473767 |
| [8] | Liu Y, Zhang XY, Fan YY, et al. Genistein reverses the effect of 17β-estradiol on exacerbating experimental occlusal interference-induced chronic masseter hyperalgesia in ovariectomised rats[J]. J Oral Rehabil, 2021, 2021, 6 (2021-06-02) [2021-09-19]. . |
| [9] | Niu K, Saloman JL, Zhang Y, et al. Sex differences in the contribution of ATP-sensitive K(+) channels in trigeminal ganglia under an acute muscle pain condition[J]. Neuroscience, 2011, 1809(4):344-352. |
| [10] |
Cairns BE, Hu JW, Arendt-Nielsen L, et al. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle[J]. J Neurophysiol, 2001, 86(2):782-791.
pmid: 11495950 |
| [11] |
Wood PB, Ledbetter CR, Glabus MF, et al. Hippocampal meta-bolite abnormalities in fibromyalgia: correlation with clinical features[J]. J Pain, 2009, 10(1):47-52.
doi: 10.1016/j.jpain.2008.07.003 |
| [12] | Shimo K, Ueno T, Younger J, et al. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain[J]. PLoS One, 2011, 6(11):1-6. |
| [13] |
Sakiyama Y, Sato A, Senda M, et al. Positron emission tomography reveals changes in global and regional cerebral blood flow during noxious stimulation of normal and inflamed elbow joints in anesthetized cats[J]. Exp Brain Res, 1998, 118(4):439-446.
doi: 10.1007/s002210050300 |
| [14] |
Derbyshire SWG, Jones AKP, Gyulai F, et al. Pain processing during three levels of noxious stimulation produces differential patterns of central activity[J]. Pain, 1997, 73(3):431-445.
pmid: 9469535 |
| [15] |
Aloisi AM, Zimmermann M, Herdegen T. Sex-dependent effects of formalin and restraint on c-Fos expression in the septum and hippocampus of the rat[J]. Neuroscience, 1997, 81(4):951-958.
pmid: 9330358 |
| [16] |
Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease[J]. Best Pract Res Clin Endocrinol Metab, 2015, 29(4):557-568.
doi: 10.1016/j.beem.2015.04.008 |
| [17] |
Warfvinge K, Krause DN, Maddahi A, et al. Estrogen receptors α, β and GPER in the CNS and trigeminal system-molecular and functional aspects[J]. J Headache Pain, 2020, 21(1):131.
doi: 10.1186/s10194-020-01197-0 |
| [18] |
Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus[J]. Neuroscience, 2006, 138(3):765-772.
pmid: 16324798 |
| [19] |
Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: A retrospective study of single-trial fMRI data[J]. Neuroimage, 2008, 39(4):1867-1876.
pmid: 18069004 |
| [20] | Hubbard CS, Karpowicz JM, Furman AJ, et al. Estrogen-depen-dent visceral hypersensitivity following stress in rats: an fMRI study[J]. Mol Pain, 2016, 12:1-10. |
| [21] |
Jie HF, Yang GJ, Bi RY, et al. Genistein antagonizes 17β-estradiol effects on glutamate-evoked masseter muscle hypernociception in rats[J]. Front Neurol, 2018, 9:649.
doi: 10.3389/fneur.2018.00649 |
| [22] | Liverman CS, Brown JW, Sandhir R, et al. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia[J]. Cephalal-gia, 2009, 29(5):520-531. |
| [23] |
Ji RR, Kohno T, Moore KA, et al. Central sensitization and LTP: do pain and memory share similar mechanisms?[J]. Trends Neurosci, 2003, 26(12):696-705.
doi: 10.1016/j.tins.2003.09.017 |
| [24] |
Martuscello RT, Spengler RN, Bonoiu AC, et al. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms[J]. Pain, 2012, 153(9):1871-1882.
doi: 10.1016/j.pain.2012.05.028 pmid: 22770843 |
| [25] |
Ding TT, Xu XX, Cao Y, et al. Inflammatory pain memory facilitates occlusal interference-induced masticatory muscle hyperalgesia in rats[J]. Eur J Pain, 2016, 20(3):353-364.
doi: 10.1002/ejp.730 pmid: 26014463 |
| [26] |
Gurtskaia G, Tsiklauri N, Nozadze I, et al. Antinociceptive tolerance to NSAIDs microinjected into dorsal hippocampus[J]. BMC Pharmacol Toxicol, 2014, 15(1):10.
doi: 10.1186/2050-6511-15-10 |
| [27] |
Mckenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test[J]. Exp Neurol, 2001, 172(1):92-99.
doi: 10.1006/exnr.2001.7777 |
| [28] |
Gol A, Faibish GM. Effects of human hippocampal ablation[J]. J Neurosurg, 1967, 26(4):390.
pmid: 6021342 |
| [29] |
Liu Y, Xu XX, Cao Y, et al. 17β-Estradiol exacerbated experimental occlusal interference-induced chronic masseter hyperalgesia by increasing the neuronal excitability and TRPV1 function of trigeminal ganglion in ovariectomized rats[J]. Int J Mol Sci, 2021, 22(13):6945.
doi: 10.3390/ijms22136945 |
| [30] | Bi RY, Meng Z, Zhang P, et al. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TM[J]. PLoS One, 2017, 12(6):1-19. |
| [31] |
Payrits M, Sághy é, Cseko K, et al. Estradiol sensitizes the transient receptor potential vanilloid 1 receptor in pain responses[J]. Endocrinology, 2017, 158(10):3249-3258.
doi: 10.1210/en.2017-00101 pmid: 28977586 |
| [32] | 徐啸翔, 曹烨, 傅开元, 等. 咬合干扰致大鼠咬肌能量代谢产物含量变化[J]. 北京大学学报(医学版), 2017, 49(1):25-30. |
| [33] | 徐啸翔, 曹烨, 丁婷婷, 等. 三叉神经节嘌呤能P2X4受体参与牙合干扰致大鼠咬肌痛觉过敏的研究[J]. 中华口腔医学杂志, 2016, 51(3):176-181. |
| [34] |
Xu XX, Cao Y, Ding TT, et al. Role of TRPV1 and ASIC3 channels in experimental occlusal interference-induced hyperalgesia in rat masseter muscle[J]. Eur J Pain, 2016, 20(4):552-563.
doi: 10.1002/ejp.758 pmid: 26201614 |
| [35] |
Chen Y, Chen AQ, Luo XQ, et al. Hippocampal NR2B-containing NMDA receptors enhance long-term potentiation in rats with chronic visceral pain[J]. Brain Res, 2014, 1570:43-53.
doi: 10.1016/j.brainres.2014.05.001 pmid: 24824341 |
| [36] | Simonic-Kocijan S, Zhao X, Liu W, et al. TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats[J]. Mol Pain, 2013, 9(1):68. |
| [37] |
Kim MT, Soussou W, Gholmieh G, et al. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3[J]. Neuroscience, 2006, 141(1):391.
pmid: 16725270 |
| [38] | 贾静. 大鼠三叉神经脊束核和海马星形胶质细胞对咬合创伤的反应[D]. 北京, 中国人民解放军军医进修学院, 2005. |
| [39] |
Wu YW, Kou XX, Bi RY, et al. Hippocampal nerve growth factor potentiated by 17β-estradiol and involved in allodynia of inflamed TMJ in rat[J]. J Pain, 2012, 13(6):555-563.
doi: 10.1016/j.jpain.2012.03.005 |
| [40] |
Leresche L, Saunders K, von Korff MR, et al. Use of exogenous hormones and risk of temporomandibular disorder pain[J]. Pain, 1997, 69(1/2):153-160.
doi: 10.1016/S0304-3959(96)03230-7 |
| [1] | Shu-dong YAN,Guang-ju YANG,Si-yi MO,Yun LIU,Qiu-fei XIE. Effect of long-term resistance exercise on masseter muscle mechanical hyperalgesia in rats [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 21-27. |
| [2] | TU Jing-yi, ZHU Ying, SHANG Shu-ling, ZHANG Xi, TANG Hui, WANG Rui-min. Keap1-tat peptide attenuates oxidative stress damage in hippocampal CA1 region and learning and memory deficits following global cerebral ischemia [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 154-159. |
|
||