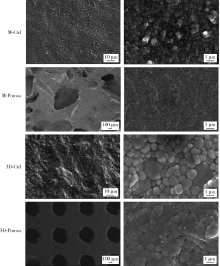
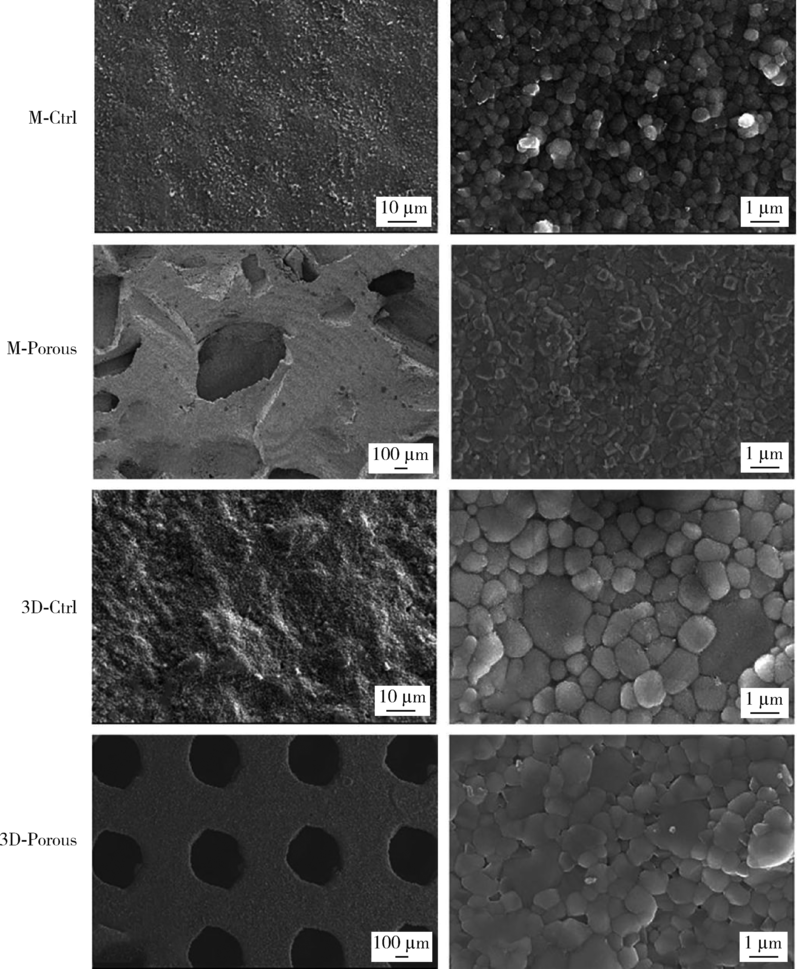
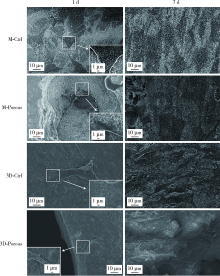
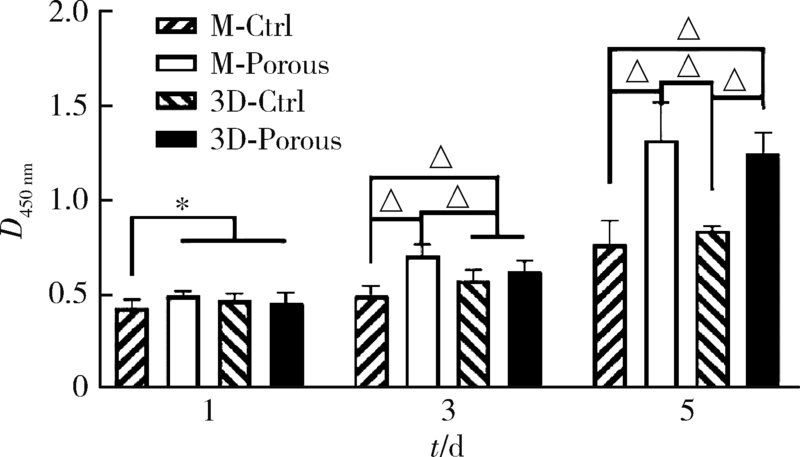
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (1): 31-39. doi: 10.19723/j.issn.1671-167X.2022.01.006
Previous Articles Next Articles
Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts
WANG Zheng1,DING Qian1,2,△( ),GAO Yuan1,MA Quan-quan1,ZHANG Lei1,△(
),GAO Yuan1,MA Quan-quan1,ZHANG Lei1,△( ),GE Xi-yuan3,SUN Yu-chun1,4,XIE Qiu-fei1
),GE Xi-yuan3,SUN Yu-chun1,4,XIE Qiu-fei1
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
2. Foshan (Southern China) Institute for New Materials, Foshan 528000, Guangdong, China
3. Central Laboratory, Peking University School and Hospital of Stomatology, Beijing 100081, China
4. Center for Digital Dentistry, Peking University School and Hospital of Stomatology, Beijing 100081, China
CLC Number:
- R781.3
| [1] |
Hanawa T. Zirconia versus titanium in dentistry: a review[J]. Dent Mater J, 2020, 39(1):24-36.
doi: 10.4012/dmj.2019-172 pmid: 31666488 |
| [2] |
Yoshinari M. Future prospects of zirconia for oral implants: a review[J]. Dent Mater J, 2020, 39(1):37-45.
doi: 10.4012/dmj.2019-151 pmid: 31666487 |
| [3] |
Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, et al. In vitro biofilm formation on titanium and zirconia implant surfaces[J]. J Periodontol, 2017, 88(3):298-307.
doi: 10.1902/jop.2016.160245 pmid: 27712464 |
| [4] |
Hafezeqoran A, Koodaryan R. Effect of zirconia dental implant surfaces on bone integration: a systematic review and meta analysis[J]. Biomed Res Int, 2017, 2017:9246721.
doi: 10.1155/2017/9246721 pmid: 28299337 |
| [5] |
Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading?[J]. Periodontol 2000, 2017, 73(1):241-258.
doi: 10.1111/prd.12180 |
| [6] |
Adanez MH, Nishihara H, Att W. A systematic review and meta analysis on the clinical outcome of zirconia implant restoration complex[J]. J Prosthodont Res, 2018, 62(4):397-406.
doi: 10.1016/j.jpor.2018.04.007 |
| [7] | Roehling S, Schlegel KA, Woelfler H, et al. Zirconia compared to titanium dental implants in preclinical studies: a systematic review and meta analysis[J]. Clin Oral Implants Res, 2019, 30(5):365-395. |
| [8] |
Kohal RJ, Bachle M, Att W, et al. Osteoblast and bone tissue response to surface modified zirconia and titanium implant materials[J]. Dent Mater, 2013, 29(7):763-776.
doi: 10.1016/j.dental.2013.04.003 pmid: 23669198 |
| [9] |
El-Hadad S, Safwat EM, Sharaf NF. In vitro and in vivo, cytoto-xicity evaluation of cast functionally graded biomaterials for dental implantology[J]. Mater Sci Eng C Mater Biol Appl, 2018, 93:987-995.
doi: 10.1016/j.msec.2018.09.003 |
| [10] |
Shirazi HA, Ayatollahi MR, Asnafi A. To reduce the maximum stress and the stress shielding effect around a dental implant bone interface using radial functionally graded biomaterials[J]. Comput Methods Biomech Biomed Engin, 2017, 20(7):750-759.
doi: 10.1080/10255842.2017.1299142 |
| [11] |
Dele-Afolabi TT, Hanim MAA, Norkhairunnisa M, et al. Research trend in the development of macroporous ceramic components by pore forming additives from natural organic matters: a short review[J]. Ceram Int, 2017, 43(2):1633-1649.
doi: 10.1016/j.ceramint.2016.10.177 |
| [12] |
Hadjicharalambous C, Mygdali E, Prymak O, et al. Proliferation and osteogenic response of MC3T3-E1 preosteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria[J]. J Biomed Mater Res A, 2015, 103(11):3612-3624.
doi: 10.1002/jbm.a.35475 pmid: 25847599 |
| [13] |
Prymak O, Vagiaki LE, Buyakov A, et al. Porous zirconia/magnesia ceramics support osteogenic potential in vitro[J]. Materials (Basel), 2021, 14(4):1049.
doi: 10.3390/ma14041049 |
| [14] | 王艳芬, 牛光良, 韩建民. 氧化锆微米涂层对成骨细胞增殖和分化的影响[J]. 中华口腔医学杂志, 2018, 53(5):339-343. |
| [15] | Ali N, Safwat A, Aboushelib M. The effect of fusion sputtering surface treatment on microshear bond strength of zirconia and MDP containing resin cement[J]. Dent Mater, 2019, 35(6):107-112. |
| [16] |
Liu Y, Rath B, Tingart M, et al. Role of implants surface modification in osseointegration: a systematic review[J]. J Biomed Mater Res A, 2020, 108(3):470-484.
doi: 10.1002/jbm.a.36829 pmid: 31664764 |
| [17] |
Sakthiabirami K, Kang JH, Jang JG, et al. Hybrid porous zirconia scaffolds fabricated using additive manufacturing for bone tissue engineering applications[J]. Mater Sci Eng C Mater Biol Appl, 2021, 123:111950.
doi: 10.1016/j.msec.2021.111950 |
| [18] |
Hwa LC, Rajoo S, Noor AM, et al. Recent advances in 3D prin-ting of porous ceramics: a review[J]. Curr Opin Solid State Mater Sci, 2017, 21(6):323-347.
doi: 10.1016/j.cossms.2017.08.002 |
| [19] |
Li R, Chen H, Wang Y, et al. Performance of stereolithography and milling in fabricating monolithic zirconia crowns with different finish line designs[J]. J Mech Behav Biomed Mater, 2021, 115:104255.
doi: 10.1016/j.jmbbm.2020.104255 |
| [20] |
Chen ZW, Li ZY, Li JJ, et al. 3D printing of ceramics: a review[J]. J Eur Ceram Soc, 2019, 39(4):661-687.
doi: 10.1016/j.jeurceramsoc.2018.11.013 |
| [21] |
Zaharin HA, Rani AMA, Azam FI, et al. Effect of unit cell type and pore size on porosity and mechanical behavior of additively manufactured Ti6Al4V scaffolds[J]. Materials (Basel), 2018, 11(12):2402.
doi: 10.3390/ma11122402 |
| [22] |
Arabnejad S, Johnston RB, Pura JA, et al. High-strength porous biomaterials for bone replacement: a strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints[J]. Acta Biomater, 2016, 30:345-356.
doi: S1742-7061(15)30177-X pmid: 26523335 |
| [23] |
Bael SV, Chai YC, Truscello S, et al. The effect of pore geometry on the in vitro biological behavior of human periosteum derived cells seeded on selective laser melted Ti6Al4V bone scaffolds[J]. Acta Biomater, 2012, 8(7):2824-2834.
doi: 10.1016/j.actbio.2012.04.001 pmid: 22487930 |
| [24] |
Wang XJ, Xu SQ, Zhou SW, et al. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review[J]. Biomaterials, 2016, 83:127-141.
doi: 10.1016/j.biomaterials.2016.01.012 |
| [25] |
Hadjicharalambous C, Buyakov A, Buyakova S, et al. Porous alumina, zirconia and alumina/zirconia for bone repair: fabrication, mechanical and in vitro biological response[J]. Biomed Mater, 2015, 10(2):025012.
doi: 10.1088/1748-6041/10/2/025012 |
| [26] |
Wang H, Su KX, Su LZ, et al. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: a biomechanical evaluation[J]. J Mech Behav Biomed Mater, 2018, 88:488-496.
doi: S1751-6161(18)30687-8 pmid: 30223212 |
| [27] | 赖颖真, 卢薛冠, 蔡艺煌. 钛及氧化锆表面微沟槽结构对人牙龈成纤维细胞生物学行为的影响[J]. 中华口腔医学杂志, 2019, 54(10):676-682. |
| [28] |
Wang C, Xu DL, Li SJ, et al. Effect of pore size on the physicochemical properties and osteogenesis of Ti6Al4V porous scaffolds with bionic structure[J]. ACS Omega, 2020, 5(44):28684-28692.
doi: 10.1021/acsomega.0c03824 |
| [29] |
Kapat K, Srivas PK, Rameshbabu AP, et al. Influence of porosity and pore size distribution in Ti6Al4V foam on physicomechanical properties, osteogenesis, and quantitative validation of bone ingrowth by micro computed tomography[J]. ACS Appl Mater Interfaces, 2017, 9(45):39235-39248.
doi: 10.1021/acsami.7b13960 |
| [30] |
Gitten RA, McLachlan T, Olivares-Navarrete R, et al. The effects of combined micron/submicron scale surface roughness and nanoscale features on cell proliferation and differentiation[J]. Biomaterials, 2011, 32(13):3395-3403.
doi: 10.1016/j.biomaterials.2011.01.029 |
| [31] |
Wang XK, Schwartz Z, Gittens RA, et al. Role of integrin alpha2 beta1 in mediating osteoblastic differentiation on three dimensional titanium scaffolds with submicron scale texture[J]. J Biomed Mater Res A, 2015, 103(6):1907-1918.
doi: 10.1002/jbm.a.v103.6 |
| [32] |
Lv J, Jia ZJ, Li J, et al. Electron beam melting fabrication of porous Ti6Al4V scaffolds: cytocompatibility and osteogenesis[J]. Adv Eng Mater, 2015, 17(9):1391-1398.
doi: 10.1002/adem.v17.9 |
| [33] |
Rezaei NM, Hasegawa M, Ishijima M, et al. Biological and osseointegration capabilities of hierarchically (meso/micro/nano scale) roughened zirconia[J]. Int J Nanomedicine, 2018, 13:3381-3395.
doi: 10.2147/IJN |
| [34] |
Mostafa D, Aboushelib M. Bioactive-hybrid-zirconia implant surface for enhancing osseointegration: an in vivo study[J]. Int J Implant Dent, 2018, 4(1):20.
doi: 10.1186/s40729-018-0129-3 pmid: 29900480 |
| [35] |
Xing HY, Zou B, Li SS, et al. Study on surface quality, precision and mechanical properties of 3D printed ZrO2 ceramic components by laser scanning stereolithography[J]. Ceram Int, 2017, 43(18):16340-16347.
doi: 10.1016/j.ceramint.2017.09.007 |
| [36] |
Zhu YL, Zhu RQ, Ma J, et al. In vitro cell proliferation evaluation of porous nano zirconia scaffolds with different porosity for bone tissue engineering[J]. Biomed Mater, 2015, 10(5):055009.
doi: 10.1088/1748-6041/10/5/055009 |
| [37] |
Seuba J, Deville S, Guizard C, et al. The effect of wall thickness distribution on mechanical reliability and strength in unidirectional porous ceramics[J]. Sci Technol Adv Mater, 2016, 17(1):128-135.
doi: 10.1080/14686996.2016.1140309 |
| [1] | Qian DING,Wen-jin LI,Feng-bo SUN,Jing-hua GU,Yuan-hua LIN,Lei ZHANG. Effects of surface treatment on the phase and fracture strength of yttria- and magnesia-stabilized zirconia implants [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 721-728. |
| [2] | Wei-wei LI,Hu CHEN,Yong WANG,Yu-chun SUN. Research on friction and wear behaviors of silicon-lithium spray coating on zirconia ceramics [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 94-100. |
| [3] | LI Wen-jin,DING Qian,YUAN Fu-song,Sun Feng-bo,ZHENG Jian-qiao,BAO Rui,Zhang Lei. Effects of femtosecond laser treatment on surface characteristics and flexural strength of zirconia [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 770-775. |
| [4] | WANG Li-xin , XU Xiao, NI Yao-feng, SUN Hai-tao, YU Ri-yue, WEI Shi-cheng. In vivo study of liposome-modified polyetheretherketone implant on bacteriostasis and osseointegration [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 758-763. |
| [5] | YANG Xin,LI Rong,YE Hong-qiang,CHEN Hu,WANG Yong,ZHOU Yong-sheng,SUN Yu-chun. Evaluation of fracture strength of two kinds of zirconia all-ceramic crowns with different edge compensation angles [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 402-405. |
| [6] | LI Zheng,WANG Xiao,HONG Tian-pei,WANG Hao-jie,GAO Zhan-yi,WAN Meng. Mechanism of advanced glycation end products inhibiting the proliferation of peripheral blood mononuclear cells and osteoblasts in rats [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 355-363. |
| [7] | Miao ZHENG,Ling-lu ZHAN,Zhi-qiang LIU,He-ping LI,Jian-guo TAN. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 315-320. |
| [8] | Zhi-yong△ ZHANG,Tian MENG,Quan CHEN,Wen-shu LIU,Yu-huan CHEN. Retrospective analysis of early dental implant failure [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1088-1091. |
| [9] | ZHOU Tuan-feng, WANG Xin-zhi . Clinical observation of the restoration of computer aided designed and manufactured one-piece zirconia posts and cores: a 5-year prospective follow-up study [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 680-684. |
| [10] | GAN Hong-quan, WANG Qian, ZHANG Hui, LIU Xin, DENG Hua-min, SONG Hui-ping, WANG ZHi-qiang, LI Qi-jia. Effects of RGD peptides-grafted porous tantalum on morphological change of MG63 osteoblasts-tantalum conjunctive interface and expression of osteogenesis factors [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 176-182. |
| [11] | CUI Xin-yue, TONG Dai, WANG Xin-zhi, SHEN Zhi-jian. Comparison of the translucency and color masking effect of the zirconia ceramics made by milling and gel deposition [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 85-90. |
| [12] | LIAO Yu, LIU Xiao-qiang, CHEN Li, ZHOU Jian-feng, TAN Jian-guo. Effects of different surface treatments on the zirconia-resin cement bond strength [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 53-57. |
| [13] | JIAO Yang, WANG Ji-de, DENG Jiu-peng. Effect of different surface treatments on the crystal structure and properties of zirconia [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 49-52. |
| [14] | LING Long, ZHAO Yu-ming, GE Li-hong. Impact of different degree pulpitis on cell proliferation and osteoblastic differentiation of dental pulp stem cell in Beagle immature premolars [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 878-883. |
| [15] | ZHOU Tuan-Feng, ZHANG Xiang-Hao, WANG Xin-Zhi. Three-dimensional finite element analysis of one-piece computer aided design and computer aided manufacture involved zirconia post and core [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 78-84. |
|
||