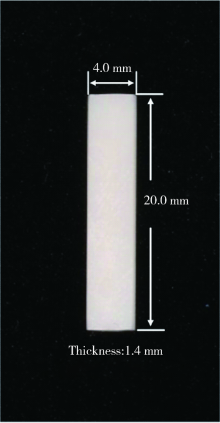
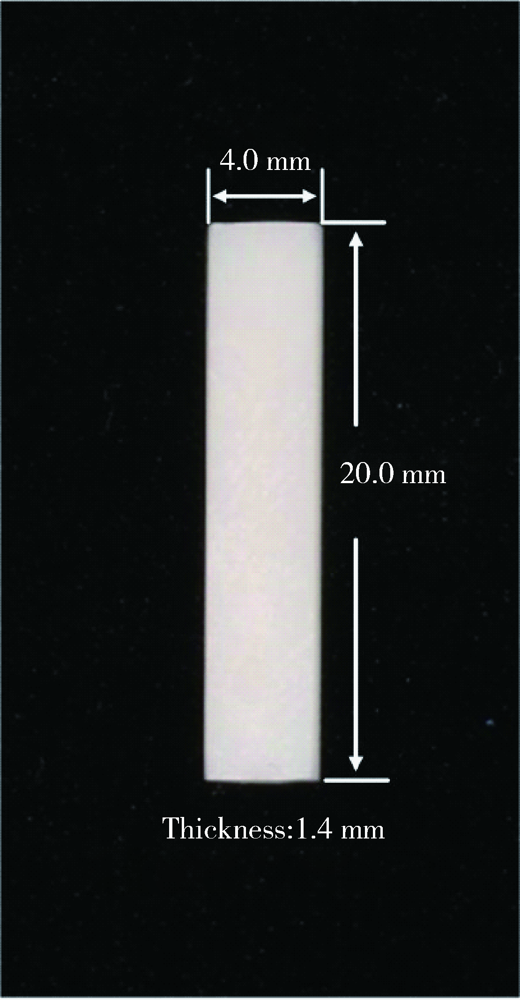
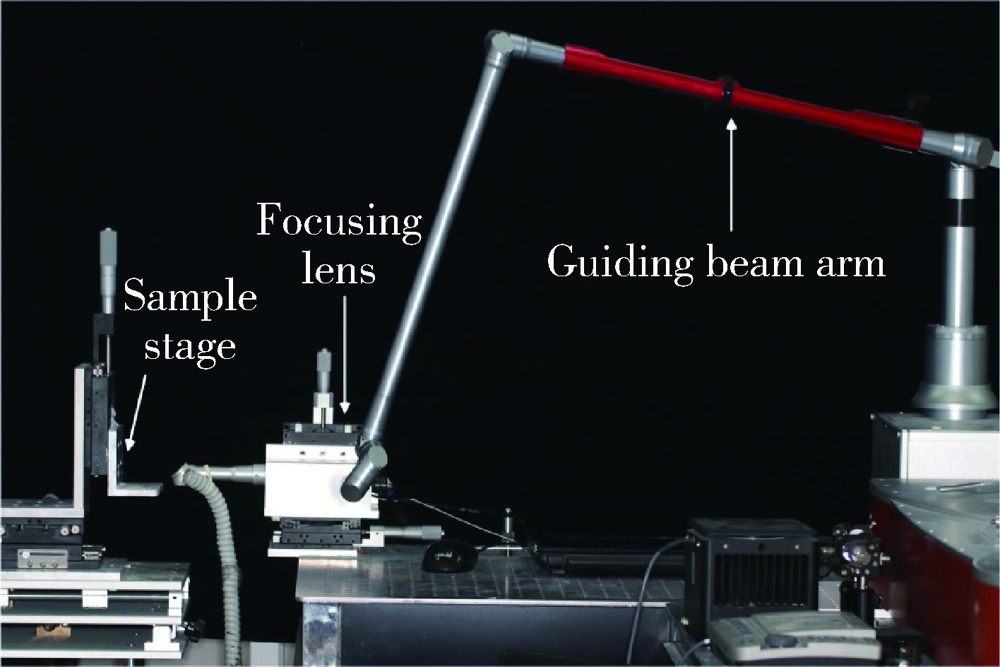
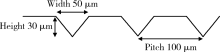
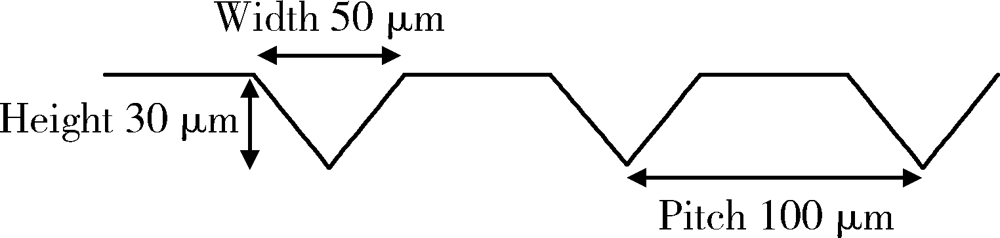
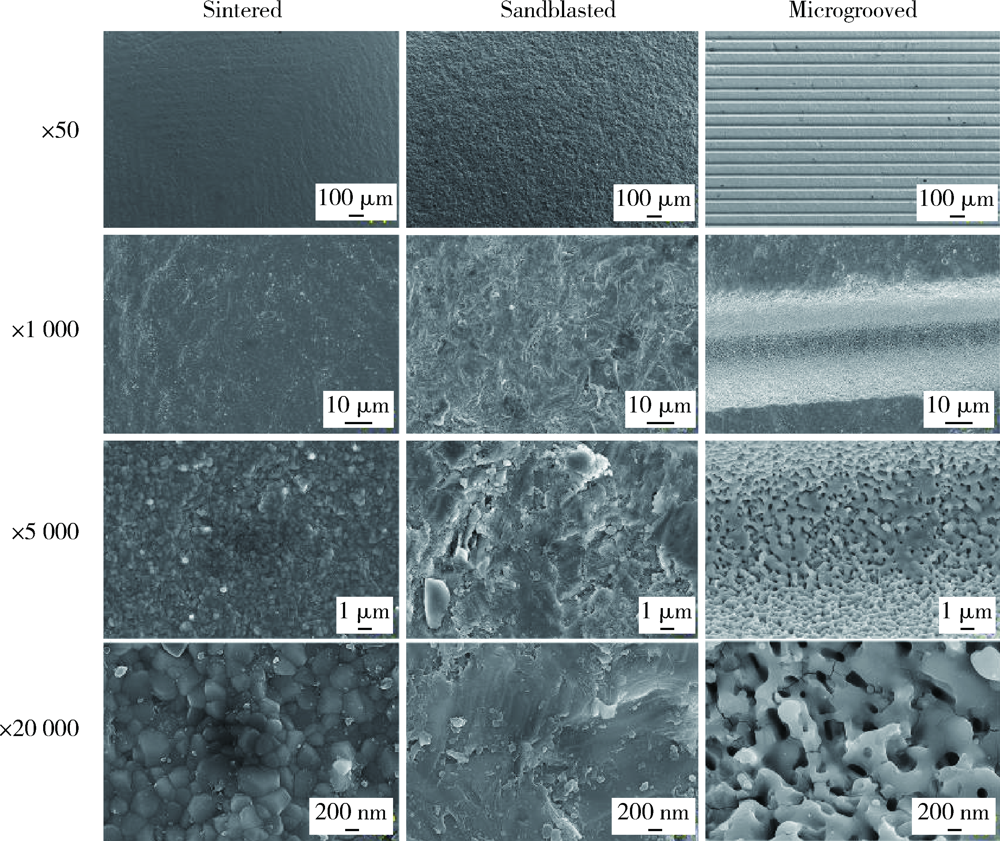
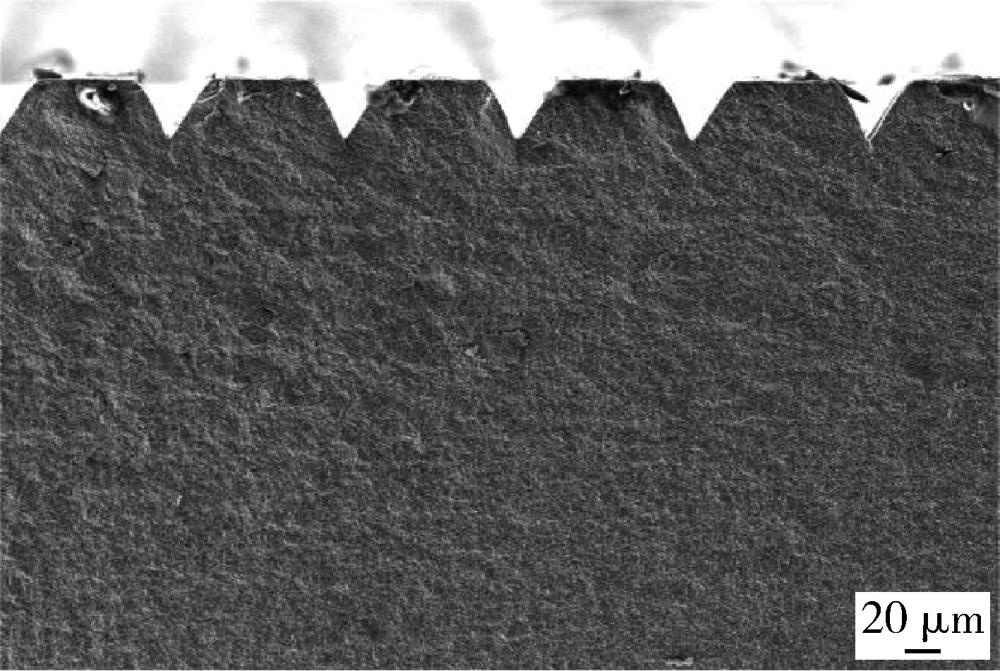
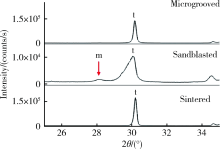
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (4): 770-775. doi: 10.19723/j.issn.1671-167X.2021.04.025
Previous Articles Next Articles
Effects of femtosecond laser treatment on surface characteristics and flexural strength of zirconia
LI Wen-jin1,DING Qian1,YUAN Fu-song2,Sun Feng-bo3,ZHENG Jian-qiao2,BAO Rui4,Zhang Lei1,Δ( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
2. Center for Digital Dentistry, Peking University School and Hospital of Stomatology & Beijing Key Laboratory of Digital Stomatology & Research Center of Engineering and Technology for Computerized Dentistry Ministry of Health, Beijing 100081, China
3. School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
4. School of Aeronautic Science and Engineering, Beihang University, Beijing 100191, China
CLC Number:
- R783.4
| [1] |
Scarano A, di Carlo F, Quaranta M, et al. Bone response to zirconia ceramic implants: an experimental study in rabbits [J]. J Oral Implantol, 2003, 29(1):8-12.
doi: 10.1563/1548-1336(2003)029<0008:BRTZCI>2.3.CO;2 |
| [2] |
Spies BC, Nold J, Vach K, et al. Two-piece zirconia oral implants withstand masticatory loads: An investigation in the artificial mouth [J]. J Mech Behav Biomed Mater, 2016, 53:1-10.
doi: 10.1016/j.jmbbm.2015.07.005 |
| [3] |
Scarano A, Piattelli M, Caputi S, et al. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study [J]. J Periodontol, 2004, 75(2):292-296.
doi: 10.1902/jop.2004.75.2.292 |
| [4] |
Borgonovo AE, Censi R, Vavassori V, et al. Zirconia implants in esthetic areas: 4-year follow-up evaluation study [J]. Int J Dent, 2015, 2015:415029.
doi: 10.1155/2015/415029 pmid: 26124836 |
| [5] | Fischer J, Schott A, Martin S. Surface micro-structuring of zirconia dental implants [J]. Clin Oral Implants Res, 2016, 27(2):162-166. |
| [6] |
Pardun K, Treccani L, Volkmann E, et al. Magnesium-containing mixed coatings on zirconia for dental implants: mechanical characterization and in vitro behavior [J]. J Biomater Appl, 2015, 30(1):104-118.
doi: 10.1177/0885328215572428 |
| [7] |
Delgado-Ruíz RA, Calvo-Guirado JL, Moreno P, et al. Femto-second laser microstructuring of zirconia dental implants [J]. J Biomed Mater Res B, 2011, 96(1):91-100.
doi: 10.1002/jbm.b.31743 pmid: 21061361 |
| [8] |
Delgado-Ruíz RA, Gomez MG, Aguilar-Salvatierra A, et al. Human fetal osteoblast behavior on zirconia dental implants and zirconia disks with microstructured surfaces. An experimental in vitro study [J]. Clin Oral Implants Res, 2016, 27(11):e144-e153.
doi: 10.1111/clr.12585 |
| [9] |
Delgado-Ruiz RA, Abboud M, Romanos G, et al. Peri-implant bone organization surrounding zirconia-microgrooved surfaces circularly polarized light and confocal laser scanning microscopy study [J]. Clin Oral Implants Res, 2015, 26(11):1328-1337.
doi: 10.1111/clr.2015.26.issue-11 |
| [10] | Pilkey WD, Pilkey DF. Peterson’s stress concentration factors[M]. 3rd ed. New Jersey: John Wiley & Sons, 2008: 3-9. |
| [11] |
Garvie RC, Nicholson PS. Phase analysis in zirconia systems [J]. J Am Ceram Soc, 1972, 55(6):303-305.
doi: 10.1111/jace.1972.55.issue-6 |
| [12] | Toraya H, Yoshimura M, Somiya S. Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction [J]. J Am Ceram Soc, 1984, 67(6):119-121. |
| [13] | British Standards Institution. ISO 6872: 2015, Dentistry-ceramic materials[S]. London: BSI Standards Publication, 2015. |
| [14] |
Quinn JB, Quinn GD. A practical and systematic review of Weibull statistics for reporting strengths of dental materials [J]. Dent Mater, 2009, 26(2):135-147.
doi: 10.1016/j.dental.2009.09.006 |
| [15] |
Kurella A, Dahotre NB. Review paper: surface modification for bioimplants: the role of laser surface engineering [J]. J Biomater Appl, 2005, 20(1):5-50.
pmid: 15972362 |
| [16] | 孙玉春, Vorobyev A, 刘晶, 等. 飞秒激光切削牙齿硬组织表面粗糙度和显微形貌观察 [J]. 中华口腔医学杂志, 2012, 47(8):486-489. |
| [17] | Roitero E, Lasserre F, Anglada M, et al. A parametric study of laser interference surface patterning of dental zirconia: Effects of laser parameters on topography and surface quality [J]. Dent Mater, 2016, 33(1):e28-e38. |
| [18] |
Ricci JL, Grew JC, Alexander H. Connective-tissue responses to defined biomaterial surfaces. Ⅰ. Growth of rat fibroblast and bone marrow cell colonies on microgrooved substrates [J]. J Biomed Mater Res A, 2008, 85A(2):313-325.
doi: 10.1002/(ISSN)1552-4965 |
| [19] |
Khandaker M, Riahinezhad S, Williams WR, et al. Microgroove and collagen-poly(ε-caprolactone) nanofiber mesh coating improves the mechanical stability and osseointegration of titanium implants [J]. Nanomaterials, 2017, 7(6):145.
doi: 10.3390/nano7060145 |
| [20] |
Souza JCM, Sordi MB, Kanazawa M, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration [J]. Acta Biomater, 2019, 94:112-131.
doi: 10.1016/j.actbio.2019.05.045 |
| [21] |
Ding Q, Zhang L, Bao R, et al. Effects of different surface treatments on the cyclic fatigue strength of one-piece CAD/CAM zirconia implants [J]. J Mech Behav Biomed Mater, 2018, 84:249-257.
doi: 10.1016/j.jmbbm.2018.05.002 |
| [22] |
Frandsen CJ, Noh K, Brammer KS, et al. Hybrid micro/nano-topography of a TiO2 nanotube-coated commercial zirconia femoral knee implant promotes bone cell adhesion in vitro [J]. Mater Sci Eng C, 2013, 33(5):2752-2756.
doi: 10.1016/j.msec.2013.02.045 |
| [23] |
Weiss P, Garber B. Shape and movement of mesenchyme cells as functions of the physical structure of the medium. Contributions to a quantitative morphology [J]. PNAS, 1952, 38(3):264-280.
pmid: 16589090 |
| [24] |
Lee M, Oh N, Lee S, et al. Factors influencing osteoblast maturation on microgrooved titanium substrata [J]. Biomaterials, 2010, 31(14):3804-3815.
doi: 10.1016/j.biomaterials.2010.01.117 |
| [25] |
Carvalho A, Cangueiro L, Oliveira V, et al. Femtosecond laser microstructured Alumina toughened Zirconia: A new strategy to improve osteogenic differentiation of hMSCs [J]. Appl Surf Sci, 2018, 435:1237-1245.
doi: 10.1016/j.apsusc.2017.11.206 |
| [26] |
de Luca AC, Zink M, Weidt A, et al. Effect of microgrooved surface topography on osteoblast maturation and protein adsorption [J]. J Biomed Mater Res A, 2015, 103(8):2689-2700.
doi: 10.1002/jbm.a.v103.8 |
| [27] |
Guilardi LF, Soares P, Werner A, et al. Fatigue performance of distinct CAD/CAM dental ceramics [J]. J Mech Behav Biomed Mater, 2019, 103:103540.
doi: 10.1016/j.jmbbm.2019.103540 |
| [28] |
Iseri U, Ozkurt Z, Yalniz A, et al. Comparison of different grinding procedures on the flexural strength of zirconia [J]. J Prosthet Dent, 2012, 107(5):309-315.
doi: 10.1016/S0022-3913(12)60081-X |
| [29] |
Aboushelib MN, Wang H, Kleverlaan CJ, et al. Fatigue behavior of zirconia under different loading conditions [J]. Dent Mater, 2016, 32(7):915-920.
doi: 10.1016/j.dental.2016.03.012 pmid: 27063462 |
| [30] |
Piconi C, Maccauro G. Zirconia as a ceramic biomaterial [J]. Biomaterials, 1999, 20(1):1-25.
pmid: 9916767 |
| [31] | 龚旭, 赵信义, 张春宝, 等. 喷砂对口腔氧化锆陶瓷抗弯强度和亚临界裂纹扩展的影响 [J]. 中华口腔医学杂志, 2017, 52(7):439-442. |
| [32] |
Kosmac T, Oblak C, Jevnikar P, et al. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic [J]. Dent Mater, 1999, 15(6):426-433.
pmid: 10863444 |
| [33] |
Aivazi M, Hossein FM, Nejatidanesh F, et al. The evaluation of prepared microgroove pattern by femtosecond laser on alumina-zirconia nano-composite for endosseous dental implant application [J]. Lasers Med Sci, 2016, 31(9):1837-1843.
doi: 10.1007/s10103-016-2059-8 |
| [34] |
Chintapalli RK, Marro FG, Jimenez-Pique E, et al. Phase transformation and subsurface damage in 3Y-TZP after sandblasting [J]. Dent Mater, 2013, 29(5):566-572.
doi: 10.1016/j.dental.2013.03.005 pmid: 23537568 |
| [35] |
Karakoca S, Yilmaz H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics [J]. J Biomed Mater Res B, 2009, 91(2):930-937.
doi: 10.1002/jbm.b.31477 pmid: 19637376 |
| [36] | Taylor D. The theory of critical distances: a new perspective in fracture mechanics[M]. London:Elsevier, 2007: 8-10. |
| [1] | Qian DING,Wen-jin LI,Feng-bo SUN,Jing-hua GU,Yuan-hua LIN,Lei ZHANG. Effects of surface treatment on the phase and fracture strength of yttria- and magnesia-stabilized zirconia implants [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 721-728. |
| [2] | Wei-wei LI,Hu CHEN,Yong WANG,Yu-chun SUN. Research on friction and wear behaviors of silicon-lithium spray coating on zirconia ceramics [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 94-100. |
| [3] | WANG Zheng,DING Qian,GAO Yuan,MA Quan-quan,ZHANG Lei,GE Xi-yuan,SUN Yu-chun,XIE Qiu-fei. Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 31-39. |
| [4] | YANG Xin,LI Rong,YE Hong-qiang,CHEN Hu,WANG Yong,ZHOU Yong-sheng,SUN Yu-chun. Evaluation of fracture strength of two kinds of zirconia all-ceramic crowns with different edge compensation angles [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 402-405. |
| [5] | Miao ZHENG,Ling-lu ZHAN,Zhi-qiang LIU,He-ping LI,Jian-guo TAN. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 315-320. |
| [6] | ZHOU Tuan-feng, WANG Xin-zhi . Clinical observation of the restoration of computer aided designed and manufactured one-piece zirconia posts and cores: a 5-year prospective follow-up study [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 680-684. |
| [7] | CUI Xin-yue, TONG Dai, WANG Xin-zhi, SHEN Zhi-jian. Comparison of the translucency and color masking effect of the zirconia ceramics made by milling and gel deposition [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 85-90. |
| [8] | LIAO Yu, LIU Xiao-qiang, CHEN Li, ZHOU Jian-feng, TAN Jian-guo. Effects of different surface treatments on the zirconia-resin cement bond strength [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 53-57. |
| [9] | JIAO Yang, WANG Ji-de, DENG Jiu-peng. Effect of different surface treatments on the crystal structure and properties of zirconia [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 49-52. |
| [10] | ZHOU Tuan-Feng, ZHANG Xiang-Hao, WANG Xin-Zhi. Three-dimensional finite element analysis of one-piece computer aided design and computer aided manufacture involved zirconia post and core [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 78-84. |
| [11] | 吕Pin , JIANG Ting. Effect of surface hot-etching treatment on zirconia/resin bonding strength [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 302-305. |
| [12] | LIU Yi-hong, WANG Yong, ZHANG Qing-hui, GAO Yuan, FENG Hai-lan. Fracture reliability of zirconia all-ceramic crown according to zirconia coping design [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 71-75. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 255
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 749
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||