Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (6): 1141-1150. doi: 10.19723/j.issn.1671-167X.2022.06.014
Previous Articles Next Articles
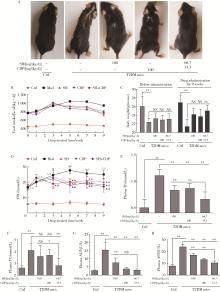
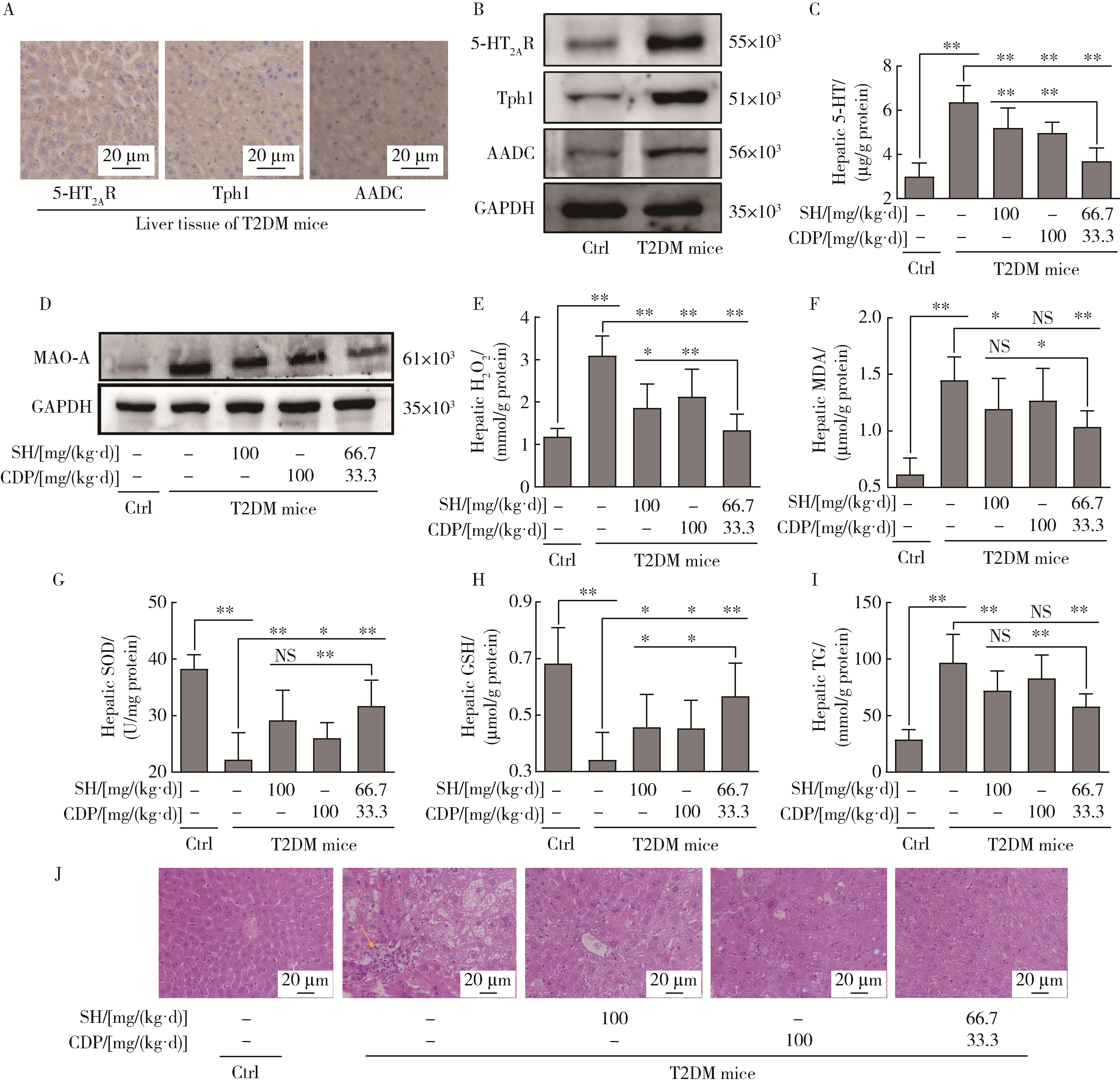
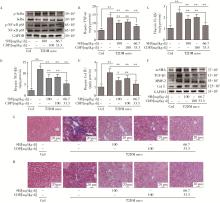
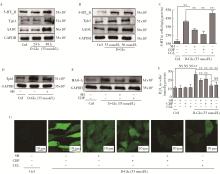
Role of hyperglycemia-induced 5-hydroxytryptamine degradation of hepatic stellate cells in hepatic inflammation and fibrosis induced by type 2 diabetes mellitus
Xiu-rui LIANG1,Xue-chun SHAN1,Jing GUAN1,Rui ZHANG1,Jing YANG1,Yi ZHANG1,Jia-qi JIN1,Yu-xin ZHANG1,Fan XU1,Ji-hua FU2,*( )
)
- 1. College of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 210009, China
2. Department of Physiology, College of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 210009, China
CLC Number:
- R363
| 1 |
Younossi ZM , Golabi P , de Avila L , et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis[J]. J Hepatol, 2019, 71 (4): 793- 801.
doi: 10.1016/j.jhep.2019.06.021 |
| 2 | Ban CR , Twigg SM . Fibrosis in diabetes complications: Pathoge-nic mechanisms and circulating and urinary markers[J]. Vasc Health Risk Manag, 2008, 4 (3): 575- 596. |
| 3 |
Gaspar P , Cases O , Maroteaux L . The developmental role of serotonin: News from mouse molecular genetics[J]. Nat Rev Neuro-sci, 2003, 4 (12): 1002- 1012.
doi: 10.1038/nrn1256 |
| 4 | Murphy DL , Lesch KP . Targeting the murine serotonin transporter: Insights into human neurobiology[J]. Nat Rev Neurosci, 2008, 9 (2): 85- 96. |
| 5 |
Keszthelyi D , Troost FJ , Masclee AAM . Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function[J]. Neurogastroenterol Motil, 2009, 21 (12): 1239- 1249.
doi: 10.1111/j.1365-2982.2009.01370.x |
| 6 |
Nickel AG , von Hardenberg A , Hohl M , et al. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure[J]. Cell Metab, 2015, 22 (3): 472- 484.
doi: 10.1016/j.cmet.2015.07.008 |
| 7 | Boess FG , Martin IL . Molecular biology of 5-HT receptors[J]. Neuropharmacology, 1994, 33 (3/4): 275- 317. |
| 8 |
Tsuchida T , Lee YA , Fujiwara N , et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer[J]. J Hepatol, 2018, 69 (2): 385- 395.
doi: 10.1016/j.jhep.2018.03.011 |
| 9 |
Nocito A , Dahm F , Jochum W , et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis[J]. Gastroenterology, 2007, 133 (2): 608- 618.
doi: 10.1053/j.gastro.2007.05.019 |
| 10 |
Knockaert L , Berson A , Ribault C , et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver[J]. Lab Invest, 2012, 92 (3): 396- 410.
doi: 10.1038/labinvest.2011.193 |
| 11 |
Kim DC , Jun DW , Kwon YI , et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis[J]. Liver Int, 2013, 33 (4): 535- 543.
doi: 10.1111/liv.12110 |
| 12 |
Fu JH , Li C , Zhang GL , et al. Crucial roles of 5-HT and 5-HT2 receptor in diabetes-related lipid accumulation and pro-inflammatory cytokine generation in hepatocytes[J]. Cell Physiol Biochem, 2018, 48 (6): 2409- 2428.
doi: 10.1159/000492656 |
| 13 |
Fu JH , Ma SX , Li X , et al. Long-term stress with hyperglucocorticoidemia-induced hepatic steatosis with VLDL overproduction is dependent on both 5-HT2 receptor and 5-HT synthesis in liver[J]. Int J Biol Sci, 2016, 12 (2): 219- 234.
doi: 10.7150/ijbs.13062 |
| 14 |
Ma SX , Li T , Guo KK , et al. Effective treatment with combination of peripheral 5-hydroxytryptamine synthetic inhibitor and 5-hydroxytryptamine 2 receptor antagonist on glucocorticoid-induced whole-body insulin resistance with hyperglycemia[J]. J Diabetes Investig, 2016, 7 (6): 833- 844.
doi: 10.1111/jdi.12526 |
| 15 | Li X , Guo KK , Li T , et al. 5-HT 2 receptor mediates high-fat diet-induced hepatic steatosis and very low density lipoprotein overproduction in rats[J]. Obes Res Clin Pract, 2018, 12 (Suppl 2): 16- 28. |
| 16 |
Zhang YX , Li C , Liang XR , et al. Role of 5-HT degradation in acute liver injury induced by carbon tetrachloride[J]. Eur J Pharmacol, 2021, 908, 174355.
doi: 10.1016/j.ejphar.2021.174355 |
| 17 |
Zhang YX , Liang XR , Guan J , et al. Carbon tetrachloride induced mitochondrial division, respiratory chain damage, abnormal intracellular [H+] and apoptosis are due to the activation of 5-HT degradation system in hepatocytes[J]. Toxicol Appl Pharmacol, 2022, 439, 115929.
doi: 10.1016/j.taap.2022.115929 |
| 18 | Schuppan D , Pinzani M . Anti-fibrotic therapy: Lost in translation?[J]. J Hepatol, 2012, 56 (Suppl 1): S66- S74. |
| 19 |
Park SY , Shin HW , Lee KB , et al. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in thioacetamide-induced chronic liver injury[J]. J Korean Med Sci, 2010, 25 (4): 570- 576.
doi: 10.3346/jkms.2010.25.4.570 |
| 20 |
Zhang CY , Yuan WG , He P , et al. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets[J]. World J Gastroenterol, 2016, 22 (48): 10512- 10522.
doi: 10.3748/wjg.v22.i48.10512 |
| 21 |
Weiskirchen R , Tacke F . Liver Fibrosis: From pathogenesis to novel therapies[J]. Dig Dis, 2016, 34 (4): 410- 422.
doi: 10.1159/000444556 |
| 22 |
Mitchell C , Couton D , Couty JP , et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice[J]. Am J Pathol, 2009, 174 (5): 1766- 1775.
doi: 10.2353/ajpath.2009.080632 |
| 23 |
Sugimoto R , Enjoji M , Kohjima M , et al. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase[J]. Liver Int, 2005, 25 (5): 1018- 1026.
doi: 10.1111/j.1478-3231.2005.01130.x |
| 24 |
Shih JC , Chen K , Ridd MJ . Monoamine oxidase: from genes to behavior[J]. Annu Rev Neurosci, 1999, 22, 197- 217.
doi: 10.1146/annurev.neuro.22.1.197 |
| 25 |
Farzanegi P , Dana A , Ebrahimpoor Z , et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation[J]. Eur J Sport Sci, 2019, 19 (7): 994- 1003.
doi: 10.1080/17461391.2019.1571114 |
| 26 |
Ruddell RG , Mann DA , Ramm GA . The function of serotonin within the liver[J]. J Hepatol, 2008, 48 (4): 666- 675.
doi: 10.1016/j.jhep.2008.01.006 |
| 27 | Papadimas GK , Tzirogiannis KN , Mykoniatis MG , et al. The emerging role of serotonin in liver regeneration[J]. Swiss Med Wkly, 2012, 142, w13548. |
| 28 |
Choi W , Namkung J , Hwang I , et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis[J]. Nat Commun, 2018, 9 (1): 4824.
doi: 10.1038/s41467-018-07287-7 |
| [1] | Chang SHU,Ye HAN,Yuzhe SUN,Zaimu YANG,Jianxia HOU. Changes of parameters associated with anemia of inflammation in patients with stage Ⅲ periodontitis before and after periodontal initial therapy [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 45-50. |
| [2] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [3] | Chen-guang ZHANG,Xu-yan CHEN,Sheng WU,Li-li FENG,Yan WANG,Yu CHEN,Min DUAN,Ke WANG,Lin-lin SONG. Internal carotid artery pseudoaneurysm caused by parapharyngeal abscess: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1135-1138. |
| [4] | Lei WANG,Xiang-shu JIN,Hui-jun DONG,Guo-min OU,Xin-yuan LAI,Hui ZHUANG,Tong LI,Kuan-hui XIANG. Establishment of a reporter system for estimating activation of human hepatic stellate cells based on COL1A1 promoter and enhanced green fluorescent protein [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 876-885. |
| [5] | CHEN Huai-an,LIU Shuo,LI Xiu-jun,WANG Zhe,ZHANG Chao,LI Feng-qi,MIAO Wen-long. Clinical value of inflammatory biomarkers in predicting prognosis of patients with ureteral urothelial carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 302-307. |
| [6] | YANG Lin-cheng,ZHANG Rui-tao,GUO Li-jun,XIAO Han,ZU Ling-yun,ZHANG You-yi,CHENG Qin,ZHAO Zhi-ling,GE Qing-gang,GAO Wei. Hypoxia and inflammation are risk factors for acute myocardial injury in patients with coronavirus disease 2019 [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 159-166. |
| [7] | Yan XUAN,Yu CAI,Xiao-xuan WANG,Qiao SHI,Li-xin QIU,Qing-xian LUAN. Effect of Porphyromonas gingivalis infection on atherosclerosis in apolipoprotein-E knockout mice [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 743-749. |
| [8] | Li-ping DUAN,Zhao-xia ZHENG,Yu-hui ZHANG,Jie DONG. Association of malnutrition-inflammation-cardiovascular disease with cognitive deterioration in peritoneal dialysis patients [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 510-518. |
| [9] | Ping WANG,Jing SONG,Xiang-yu FANG,Xin LI,Xu LIU,Yuan JIA,Zhan-guo LI,Fan-lei HU. Role of erythroblast-like Ter cells in the pathogenesis of collagen-induced arthritis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 445-450. |
| [10] | GAI Xiao-yan, CHANG Chun, WANG Juan, LIANG Ying, LI Mei-jiao, SUN Yong-chang,HE Bei, YAO Wan-zhen. Airway inflammation and small airway wall remodeling in neutrophilic asthma [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 645-650. |
| [11] | FAN Ai-qin, FENG Jin-qiu, LIU Wei, ZHANG Min-jia, LIU Tan, ZHOU Ya-lin, XU Ya-jun. Antagonistic effect of quercetin on PM2.5 toxicity in the rat’s embryonic development in vitro [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 388-393. |
| [12] | ZHU Hui, ZHU Rong, DENG Zhao-da, FENG Yu-chen, SHEN Hai-li. Analgesic effects of ionotropic glutamate receptor antagonists MK-801 and NBQX on collagen-induced arthritis rats [J]. Journal of Peking University(Health Sciences), 2016, 48(6): 977-981. |
| [13] | CHEN Xiao-mei, LI Fu-qiang, YAN Su, WU Xiao-cui, TANG Cui-lan. Nicotine alleviates the liver inflammation of non-alcoholic steatohepatitis induced by high-fat and high-fructose in mice [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 777-782. |
| [14] | JIA Shuang-shuang, LI Wei-yang, LIU Xin, LI Li-ying. Transforming growth factor-β1 induces differentiation of bone marrowderived mesenchymal stem cells into myofibroblasts via production of reactive oxygen species [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 737-742. |
| [15] | ZHANG Wen-Xiao, SHANG Jing, GAO Xin, LUAN Xian-Guo, LI Ping, WANG Tian-Jing, HU Gui-Ping, ZHU Tong, JIA Guang. Effect of 1,4-naphthoquinone aged black carbon on reactive oxygen species and DNA strand breaks in human bronchial epithelial cells [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 690-696. |
|
||