Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (1): 160-166. doi: 10.19723/j.issn.1671-167X.2023.01.025
Previous Articles Next Articles
Diagnostic value of F wave changes in patients with Charcot-Marie-Tooth1A and chronic inflammatory demyelinating polyneuropathy
Xiao-xuan LIU,Shuo ZHANG,Yan MA,A-ping SUN,Ying-shuang ZHANG,Dong-sheng FAN*( )
)
- Department of Neurology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R745.4
| 1 |
Rossor AM , Polke JM , Houlden H , et al. Clinical implications of genetic advances in Charcot-Marie-Tooth disease[J]. Nat Rev Neurol, 2013, 9 (10): 562- 571.
doi: 10.1038/nrneurol.2013.179 |
| 2 | Davis CJ , Bradley WG , Madrid R . The peroneal muscular atrophy syndrome: Clinical, genetic, electrophysiological and nerve biopsy studies. Ⅰ. Clinical, genetic and electrophysiological findings and classification[J]. J Genet Hum, 1978, 26 (4): 311- 349. |
| 3 |
刘小璇, 樊东升, 宋淑娟. 中国人群腓骨肌萎缩症的致病基因分布特点及临床表型[J]. 中华内科杂志, 2015, 54 (7): 623- 627.
doi: 10.3760/cma.j.issn.0578-1426.2015.07.011 |
| 4 |
van den Bergh PY , Hadden RD , Bouche P , et al. European Fe-deration of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Fe-deration of Neurological Societies and the Peripheral Nerve Society: First revision[J]. Eur J Neurol, 2010, 17 (3): 356- 363.
doi: 10.1111/j.1468-1331.2009.02930.x |
| 5 |
马妍, 鲁明, 樊东升, 等. 无力麻木-周围神经损害-抗NF155 IgG4抗体阳性[J]. 中华医学杂志, 2016, 96 (43): 3519- 3543.
doi: 10.3760/cma.j.issn.0376-2491.2016.43.017 |
| 6 |
马妍, 鲁明, 樊东升. 抗NF155 IgG抗体阳性慢性炎性脱髓鞘性多发性神经根一例并文献复习[J]. 中国神经免疫学和神经病学杂志, 2017, 24 (3): 188- 196.
doi: 10.3969/j.issn.1006-2963.2017.03.009 |
| 7 |
马妍, 鲁明, 樊东升. 郎飞结/结旁疾病慢性炎性脱髓鞘性多发性神经根相关抗体的研究进展[J]. 中华神经科杂志, 2017, 50 (10): 778- 780.
doi: 10.3760/cma.j.issn.1006-7876.2017.10.016 |
| 8 |
Ogata H , Yamasaki R , Hiwatashi A , et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy[J]. Ann Clin Transl Neurol, 2015, 2 (10): 960- 971.
doi: 10.1002/acn3.248 |
| 9 |
Querol L , Nogales-Gadea G , Rojas-Garcia R , et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg[J]. Neurology, 2014, 82 (10): 879- 886.
doi: 10.1212/WNL.0000000000000205 |
| 10 |
Devaux JJ , Miura Y , Fukami Y , et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy[J]. Neurology, 2016, 86 (9): 800- 807.
doi: 10.1212/WNL.0000000000002418 |
| 11 |
Niu J , Cui L , Liu M , et al. Multiple sites ultrasonography of peripheral nerves in differentiating Charcot-Marie-Tooth Type 1A from chronic inflammatory demyelinating polyradiculoneuropathy[J]. Front Neurol, 2017, 8, 181.
doi: 10.3389/fneur.2017.00181 |
| 12 |
Vaeggemose M , Vaeth S , Pham M , et al. Magnetic resonance reurography and diffusion tensor imaging of the peripheral nerves in patients with CMT Type 1A[J]. Muscle Nerve, 2017, 56 (6): E78- E84.
doi: 10.1002/mus.25691 |
| 13 |
中华医学会神经病学分会, 中华医学会神经病学分会周围神经病协作组, 中华医学会神经病学分会肌电图与临床神经电生理学组, 中华医学会神经病学分会神经肌肉病学组. 中国慢性炎症性脱髓鞘性多发性神经根神经病诊治指南[J]. 中华神经科杂志, 2019, 52 (11): 883- 888.
doi: 10.3760/cma.j.issn.1006-7876.2019.11.003 |
| 14 |
Pefla L , Moreno CB , Gutierrez-Alvarez AM . Pain management in Guillain-Barre syndrome: A systematic review[J]. Neurologia, 2015, 30 (7): 433- 438.
doi: 10.1016/j.nrl.2014.04.009 |
| 15 | 汤晓芙. 临床肌电图学[M]. 北京: 北京医科大学/中国协和医科大学联合出版社, 1995: 51- 59. |
| 16 | 卢祖能. 实用肌电图学[M]. 北京: 人民卫生出版社, 2000: 851- 853. |
| 17 | Wang ZL , Liu M , Ding Q . Split-hand index in amyotrophic lateral sclerosis: An F-wave study[J]. Amyotroph Lateral Scler Frontotemporal Degener, 2019, 20 (7/8): 562- 567. |
| 18 |
Baek SH , Hong YH , Choi SJ , et al. Electrodiagnostic data-driven clustering identifies a prognostically different subgroup of patients with chronic inflammatory demyelinating polyneuropathy[J]. J Neurol Neurosurg Psychiatry, 2019, 90 (6): 674- 680.
doi: 10.1136/jnnp-2018-319758 |
| 19 |
van den Bergh PYK , van Doorn PA , et al. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force-second revision[J]. Eur J Neurol, 2021, 28 (11): 3556- 3583.
doi: 10.1111/ene.14959 |
| 20 | Martín-Aguilar L , Lleixà C , Pascual-Goñi E , et al. Clinical and laboratory features in anti-NF155 autoimmune nodopathy[J]. Neurol Neuroimmunol Neuroinflamm, 2021, 9 (1): e1098. |
| 21 |
Chhabra A , Madhuranthakam AJ , Andreisek G . Magnetic resonance neurography: Current perspectives and literature review[J]. Eur Radiol, 2018, 28 (2): 698- 707.
doi: 10.1007/s00330-017-4976-8 |
| 22 |
Kronlage M , Pitarokoili K , Schwarz D , et al. Diffusion tensor imaging in chronic inflammatory demyelinating polyneuropathy: Diagnostic accuracy and correlation with electrophysiology[J]. Invest Radiol, 2017, 52 (11): 701- 707.
doi: 10.1097/RLI.0000000000000394 |
| 23 |
Doppler K , Stengel H , Appeltshauser L , et al. Neurofascin-155 IgM autoantibodies in patients with inflammatory neuropathies[J]. J Neurol Neurosurg Psychiatry, 2018, 89 (11): 1145- 1151.
doi: 10.1136/jnnp-2018-318170 |
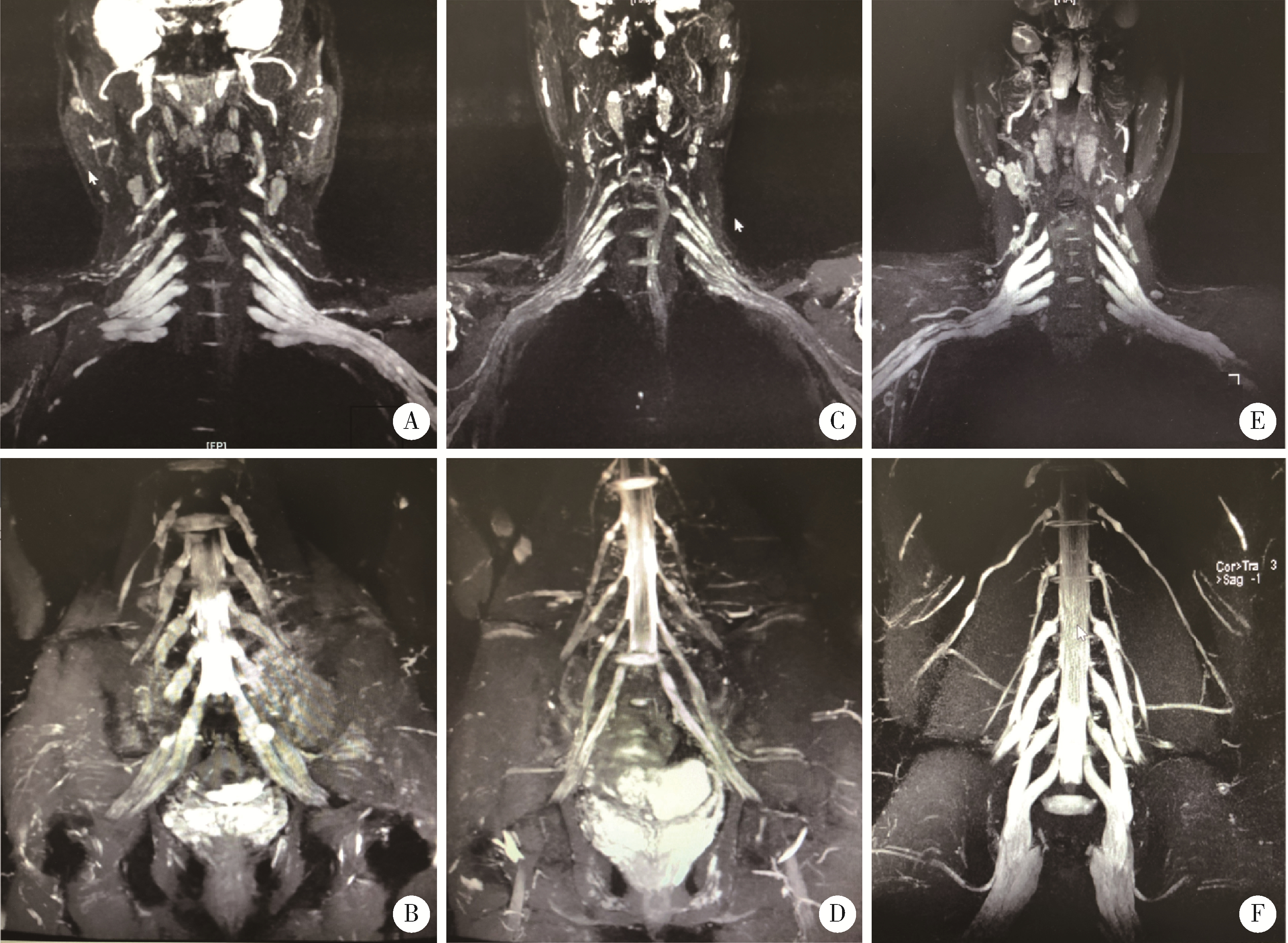
| 24 | Wu F , Ren Y , Wang WW , et al. Microstructural alteration of lumbosacral nerve roots in chronic inflammatory demyelinating polyradiculoneuropathy: Insights from DTI and correlations with electrophysiological parameters[J]. Acad Radiol, 2022, 29 (Suppl 3): 175- 182. |
| [1] | Shuhui YU,Jianing HAN,Lijun ZHONG,Congyu CHEN,Yunxiang XIAO,Yanbo HUANG,Yang YANG,Xinyan CHE. Predictive value of preoperative pelvic floor electrophysiological parameters on early urinary incontinence following radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 594-599. |
| [2] | Mei-ge LIU,Pu FANG,Yan WANG,Lu CONG,Yang-yi FAN,Yuan YUAN,Yan XU,Jun ZHANG,Dao-jun HONG. Clinical, pathological and genetic characteristics of 8 patients with distal hereditary motor neuropathy [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 957-963. |
| [3] | Guo-zhong LIN,Zhen-yu WANG,Jing-cheng XIE,Bin LIU,Chang-cheng MA,Xiao-dong CHEN. Clinical study of 21 cases of sacral cysts containing fila terminale [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 582-585. |
| [4] | Jing WANG,Jun-peng CHEN,Yang WANG,Xiang-liang XU,Chuan-bin GUO. Application of digital mandibular movement record and masticatory muscle electromyography in the evaluation of stomatognathic function in patients with mandibular tumor [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 571-578. |
|
||