Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 339-342. doi: 10.19723/j.issn.1671-167X.2023.02.020
Previous Articles Next Articles
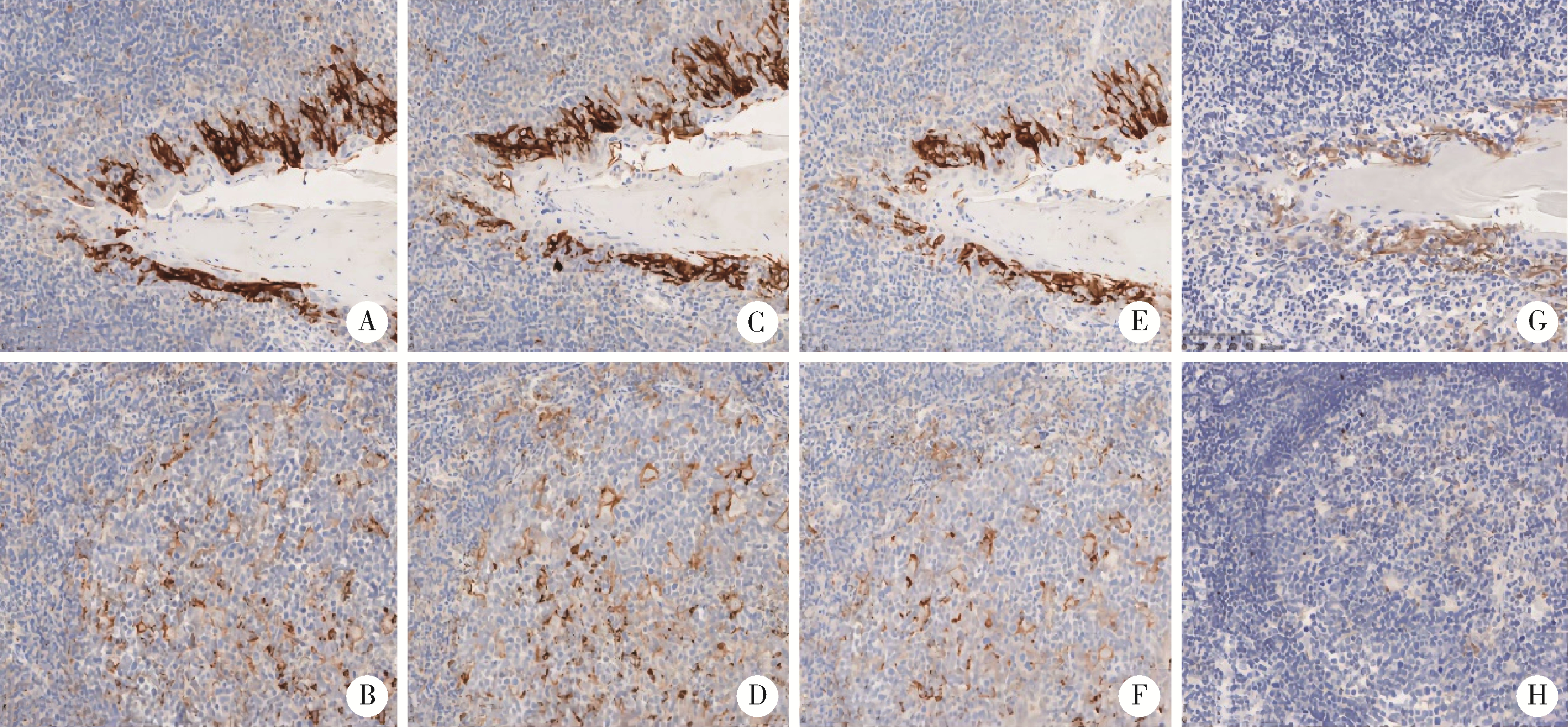
Consistency comparison of programmed cell death 1-ligand 1 in different immuno-histochemical staining methods
Dong LI,Ji-ting DI,Yan XIONG*( )
)
- Department of Pathology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R365
| 1 |
Hu-Lieskovan S , Bhaumik S , Dhodapkar K , et al. SITC cancer immunotherapy resource document: A compass in the land of biomarker discovery[J]. J Immunother Cancer, 2020, 8 (2): e000705.
doi: 10.1136/jitc-2020-000705 |
| 2 |
Parra ER , Villalobos P , Mino B , et al. Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1(PD-L1) on non small cell lung carcinoma[J]. Appl immunohistochem mol morphol, 2018, 26 (2): 83- 93.
doi: 10.1097/PAI.0000000000000531 |
| 3 |
Scorer P , Scott M , Lawson N , et al. Consistency of tumor and immune cell programmed cell death ligand-1 expression within and between tumor blocks using the VENTANA SP263 assay[J]. Diagn Pathol, 2018, 13 (1): 47.
doi: 10.1186/s13000-018-0725-9 |
| 4 |
Kim SY , Kim TE , Park CK , et al. Comprehensive comparison of 22C3 and SP263 PD-L1 expression in non small cell lung cancer using routine clinical and conditioned archives[J]. Cancers (Basel), 2022, 14 (13): 3138.
doi: 10.3390/cancers14133138 |
| 5 | Song L , Zeng L , Yan H , et al. Validation of E1L3N antibody for PD-L1 detection and prediction of pembrolizumab response in non small cell lung cancer[J]. Commun Med (Lond), 2022, 2 (1): 137. |
| 6 |
Lawson NL , Dix CI , Scorer PW , et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies[J]. Mod Pathol, 2020, 33 (4): 518- 530.
doi: 10.1038/s41379-019-0372-z |
| 7 |
Keppens C , Dequeker EM , Pauwels P , et al. PD-L1 immunohistochemistry in non small cell lung cancer: Unraveling differences in staining concordance and interpretation[J]. Virchows Arch, 2021, 478 (5): 827- 839.
doi: 10.1007/s00428-020-02976-5 |
| 8 | Xu H , Dong X , Zhao H , et al. Clinical evaluation of a laboratory-developed test using clone E1L3N for the detection of PD-L1 expression status in non small cell lung cancer[J]. J Clin Lab Anal, 2021, 35 (3): e23696. |
| 9 |
Ilie M , Khambata-Ford S , Copie-Bergman C , et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms[J]. PLoS One, 2017, 12 (8): e0183023.
doi: 10.1371/journal.pone.0183023 |
| 10 | Schalper KA , Daniel CH , Joseph M , et al. Clinical significance of PD-L1 protein expression on tumor-associated macrophages in lung cancer[J]. J Immunother Cancer, 2015, 3 (2): 415. |
| 11 |
McLaughlin J , Han G , Schalper KA , et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non small cell lung cancer[J]. JAMA Oncol, 2016, 2 (1): 46- 54.
doi: 10.1001/jamaoncol.2015.3638 |
| [1] | Yan XIONG,Bo ZHANG,Li-gong NIE,Shi-kai WU,Hu ZHAO,Dong LI,Ji-ting DI. Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 351-356. |
| [2] | YU Yan-fei,HE Shi-ming,WU Yu-cai,XIONG Sheng-wei,SHEN Qi,LI Yan-yan,YANG Feng,HE Qun,LI Xue-song. Clinicopathological features and prognosis of fumarate hydratase deficient renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 640-646. |
| [3] | CHI Yan-ting,ZHANG Yan-ping,ZHANG Qiu-lu,LIU Cui-ling,LI bin-bin. Clinicopathological analysis of mucosa associated lymphoid tissue lymphoma secondary to Sjögren’s syndrome in salivary gland [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 40-45. |
| [4] | Ru MA,Xin-bao LI,Feng-cai YAN,Yu-lin LIN,Yan LI. Clinical evaluation of tumor-stroma ratio in pseudomyxoma peritonei from the appendix [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 240-246. |
| [5] | Xiao-peng ZHANG,Wei-yu ZHANG,Fei HUO,Hao HU,Qi WANG,Ke-xin XU. Outcome of surgical management and pathogenesis of female primary bladder neck obstruction [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1052-1055. |
| [6] | Chun-feng ZHANG,Yun LIU,Min LU,Xiao-juan DU. Expression of hUTP14a in non-small cell lung cancer [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 145-150. |
| [7] | Lei LIU,Li-hua WANG,Yu-bo REN,Xiao-song RAO,Shao-min YANG. Clinicopathological analysis of aggressive angiomyxoma of soft tissue in abdomino-pelvic cavity [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1098-1101. |
| [8] | MEI Fang, ZHAO Ting-ting, GAO Fei, ZHENG Jie. A rare pulmonary benign bi-phasic tumor: a case report of pulmonary adenofibroma and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1076-1080. |
| [9] | LIU Chang, CUI Li-gang, WANG Hong-lei. Renal Ewing’s sarcoma/primitive neuroectodermal tumor: a case report and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 919-923. |
| [10] | XI Chen-guang, FAN Yu, YANG Xin-yu, LIU Li-bo, WANG Jing-hua, HU Shuai, LI Yan-yan, HE Qun. Clinicopathological features and differential diagnosis of metanephric adenoma: a report of sixteen cases [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 598-602. |
| [11] | SI Jing-wen, WANG Li, BA Xiao-jun, ZHANG Xu, DONG Ying, ZHANG Ji-xin, LI Wen-ting, LI Ting. Clinicopathological screening of Lynch syndrome: a report of 2 cases and literature review [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 858-864. |
| [12] | GONG Bei, HU Hui-Hui, ZHANG Man. Expression of apolipoprotein A-Ⅰ in eight histological types of renal neoplasms [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 155-159. |
| [13] | ZHANG Meng-Xue-1, PEI Fei-1△, WANG Tian-Li-2, HAN Xiang-3, YOU Jiang-Feng-1, ZOU Peng-Cheng-1, WANG Yue-Qi-1, LI Xu-Wen-1, LIU Xin-1, ZHONG Gao-Gao-1, LIU Yan-1, WANG Yu-Xiang-1, WANG Hua-1, ZHANG Bo-1. Anaplastic lymphoma kinase fusion gene expression, clinical pathological characteristics and prognosis in 95 Chinese patients with non-small cell lung cancer [J]. Journal of Peking University(Health Sciences), 2014, 46(4): 582-588. |
| [14] | MA Rui-qiong, CHENG Hong-yan, YE Xue, CHEN Jun, CUI Heng, WEI Li-hui, CHANG Xiao-hong. Expression and significance of tumor necrosis factor receptor associated protein 1 in epithelial ovarian cancer [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 120-124. |
|
||